You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001735_02944
You are here: Home > Sequence: MGYG000001735_02944
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
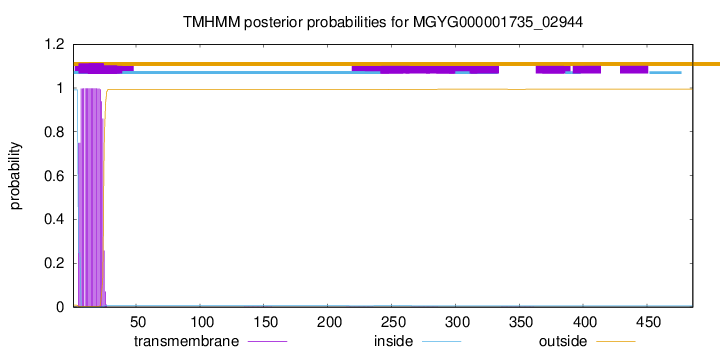
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; | |||||||||||
| CAZyme ID | MGYG000001735_02944 | |||||||||||
| CAZy Family | CE3 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 10750; End: 12210 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE3 | 34 | 222 | 3e-41 | 0.9845360824742269 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG0657 | Aes | 1.49e-32 | 245 | 478 | 60 | 312 | Acetyl esterase/lipase [Lipid transport and metabolism]. |
| pfam07859 | Abhydrolase_3 | 1.69e-29 | 268 | 453 | 1 | 205 | alpha/beta hydrolase fold. This catalytic domain is found in a very wide range of enzymes. |
| cd01833 | XynB_like | 2.49e-28 | 32 | 222 | 1 | 157 | SGNH_hydrolase subfamily, similar to Ruminococcus flavefaciens XynB. Most likely a secreted hydrolase with xylanase activity. SGNH hydrolases are a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| COG1506 | DAP2 | 2.05e-21 | 234 | 453 | 364 | 594 | Dipeptidyl aminopeptidase/acylaminoacyl peptidase [Amino acid transport and metabolism]. |
| pfam00326 | Peptidase_S9 | 2.19e-14 | 283 | 453 | 2 | 187 | Prolyl oligopeptidase family. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BAX78799.1 | 2.40e-173 | 25 | 483 | 31 | 494 |
| ATC65549.1 | 6.34e-138 | 34 | 483 | 40 | 492 |
| QOV88633.1 | 4.36e-65 | 34 | 222 | 32 | 222 |
| QDT00894.1 | 1.49e-60 | 236 | 483 | 34 | 283 |
| QNN20991.1 | 2.79e-58 | 18 | 226 | 18 | 226 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5AO9_A | 6.45e-51 | 236 | 486 | 16 | 281 | Thestructure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-native [Thermogutta terrifontis],5AOA_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-Propionate bound [Thermogutta terrifontis],5AOB_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-butyrate bound [Thermogutta terrifontis],5AOC_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-valerate bound [Thermogutta terrifontis] |
| 7BFN_A | 6.63e-51 | 236 | 486 | 17 | 282 | ChainA, Esterase [Thermogutta terrifontis] |
| 7BFO_A | 3.56e-50 | 236 | 486 | 17 | 282 | ChainA, Esterase [Thermogutta terrifontis],7BFR_A Chain A, Esterase [Thermogutta terrifontis],7BFT_A Chain A, Esterase [Thermogutta terrifontis],7BFU_A Chain A, Esterase [Thermogutta terrifontis],7BFV_A Chain A, Esterase [Thermogutta terrifontis] |
| 2YH2_A | 2.51e-13 | 246 | 354 | 60 | 164 | Pyrobaculumcalidifontis esterase monoclinic form [Pyrobaculum calidifontis],2YH2_B Pyrobaculum calidifontis esterase monoclinic form [Pyrobaculum calidifontis],2YH2_C Pyrobaculum calidifontis esterase monoclinic form [Pyrobaculum calidifontis],2YH2_D Pyrobaculum calidifontis esterase monoclinic form [Pyrobaculum calidifontis],3ZWQ_A Hyperthermophilic Esterase From The Archeon Pyrobaculum Calidifontis [Pyrobaculum calidifontis JCM 11548],3ZWQ_B Hyperthermophilic Esterase From The Archeon Pyrobaculum Calidifontis [Pyrobaculum calidifontis JCM 11548] |
| 1QZ3_A | 1.91e-12 | 246 | 453 | 57 | 282 | CRYSTALSTRUCTURE OF MUTANT M211S/R215L OF CARBOXYLESTERASE EST2 COMPLEXED WITH HEXADECANESULFONATE [Alicyclobacillus acidocaldarius],1U4N_A Crystal Structure Analysis of the M211S/R215L EST2 mutant [Alicyclobacillus acidocaldarius] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P96402 | 1.08e-11 | 266 | 453 | 157 | 367 | Esterase LipC OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=lipC PE=1 SV=1 |
| Q9US38 | 1.04e-09 | 253 | 480 | 88 | 340 | AB hydrolase superfamily protein C1039.03 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=SPAC1039.03 PE=3 SV=1 |
| Q8VYP9 | 2.24e-09 | 233 | 453 | 176 | 420 | Probable isoprenylcysteine alpha-carbonyl methylesterase ICMEL1 OS=Arabidopsis thaliana OX=3702 GN=ICMEL1 PE=2 SV=1 |
| Q84LM4 | 2.15e-08 | 187 | 455 | 460 | 741 | Acylamino-acid-releasing enzyme OS=Arabidopsis thaliana OX=3702 GN=AARE PE=1 SV=1 |
| W7MTJ1 | 9.18e-08 | 229 | 457 | 42 | 289 | Esterase FVEG_12639 OS=Gibberella moniliformis (strain M3125 / FGSC 7600) OX=334819 GN=FVEG_12639 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
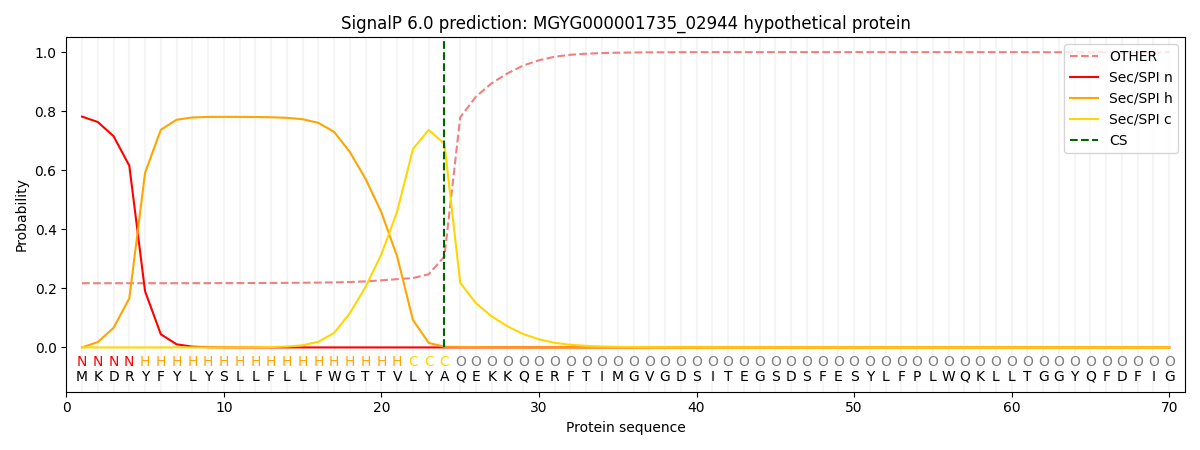
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.231333 | 0.765897 | 0.001476 | 0.000401 | 0.000389 | 0.000480 |