You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001757_00756
You are here: Home > Sequence: MGYG000001757_00756
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Ruminococcus sp900540005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus; Ruminococcus sp900540005 | |||||||||||
| CAZyme ID | MGYG000001757_00756 | |||||||||||
| CAZy Family | GH11 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 12995; End: 14626 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH11 | 42 | 240 | 7.5e-66 | 0.9887005649717514 |
| CBM22 | 268 | 397 | 6e-33 | 0.9770992366412213 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00457 | Glyco_hydro_11 | 6.47e-68 | 42 | 239 | 1 | 175 | Glycosyl hydrolases family 11. |
| pfam02018 | CBM_4_9 | 1.59e-22 | 267 | 400 | 3 | 134 | Carbohydrate binding domain. This family includes diverse carbohydrate binding domains. |
| cd14256 | Dockerin_I | 2.27e-06 | 468 | 534 | 2 | 57 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CAB51934.1 | 1.65e-174 | 1 | 543 | 1 | 514 |
| AAB26620.1 | 1.65e-174 | 1 | 543 | 1 | 514 |
| CAA90271.1 | 3.41e-143 | 1 | 413 | 1 | 410 |
| AAR39816.1 | 4.81e-143 | 3 | 413 | 2 | 410 |
| CAA84537.1 | 4.29e-142 | 1 | 479 | 1 | 456 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7AYL_A | 3.27e-72 | 1 | 248 | 1 | 230 | Crystalstructure of the GH11 domain of a multidomain xylanase from the hindgut metagenome of Trinervitermes trinervoides [uncultured bacterium],7AYL_B Crystal structure of the GH11 domain of a multidomain xylanase from the hindgut metagenome of Trinervitermes trinervoides [uncultured bacterium] |
| 2F6B_A | 2.82e-70 | 42 | 251 | 12 | 205 | Structuraland active site modification studies implicate Glu, Trp and Arg in the activity of xylanase from alkalophilic Bacillus sp. (NCL 87-6-10). [Bacillus],2F6B_B Structural and active site modification studies implicate Glu, Trp and Arg in the activity of xylanase from alkalophilic Bacillus sp. (NCL 87-6-10). [Bacillus] |
| 1H4G_A | 4.10e-70 | 42 | 251 | 12 | 205 | Oligosaccharide-bindingto family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens],1H4G_B Oligosaccharide-binding to family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens],1QH6_A CATALYSIS AND SPECIFICITY IN ENZYMATIC GLYCOSIDE HYDROLASES: A 2,5B CONFORMATION FOR THE GLYCOSYL-ENZYME INTERMIDIATE REVEALED BY THE STRUCTURE OF THE BACILLUS AGARADHAERENS FAMILY 11 XYLANASE [Salipaludibacillus agaradhaerens],1QH6_B CATALYSIS AND SPECIFICITY IN ENZYMATIC GLYCOSIDE HYDROLASES: A 2,5B CONFORMATION FOR THE GLYCOSYL-ENZYME INTERMIDIATE REVEALED BY THE STRUCTURE OF THE BACILLUS AGARADHAERENS FAMILY 11 XYLANASE [Salipaludibacillus agaradhaerens],1QH7_A CATALYSIS AND SPECIFICITY IN ENZYMATIC GLYCOSIDE HYDROLASES: A 2,5B CONFORMATION FOR THE GLYCOSYL-ENZYME INTERMIDIATE REVEALED BY THE STRUCTURE OF THE BACILLUS AGARADHAERENS FAMILY 11 XYLANASE [Salipaludibacillus agaradhaerens],1QH7_B CATALYSIS AND SPECIFICITY IN ENZYMATIC GLYCOSIDE HYDROLASES: A 2,5B CONFORMATION FOR THE GLYCOSYL-ENZYME INTERMIDIATE REVEALED BY THE STRUCTURE OF THE BACILLUS AGARADHAERENS FAMILY 11 XYLANASE [Salipaludibacillus agaradhaerens] |
| 1H4H_A | 3.39e-69 | 42 | 251 | 12 | 205 | Oligosaccharide-bindingto family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens],1H4H_B Oligosaccharide-binding to family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens],1H4H_C Oligosaccharide-binding to family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens],1H4H_D Oligosaccharide-binding to family 11 xylanases: both covalent intermediate and mutant-product complexes display 2,5B conformations at the active-centre [Salipaludibacillus agaradhaerens] |
| 2NQY_A | 2.38e-68 | 29 | 251 | 1 | 205 | Crystalstructure of alkaline thermophlic xylanase from Bacillus sp. (NCL 86-6-10) with complex xylotriose: Xylotriose cleaved to xylobiose and xylose [Bacillus sp. (in: Bacteria)],2NQY_B Crystal structure of alkaline thermophlic xylanase from Bacillus sp. (NCL 86-6-10) with complex xylotriose: Xylotriose cleaved to xylobiose and xylose [Bacillus sp. (in: Bacteria)] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q53317 | 4.66e-175 | 1 | 543 | 1 | 514 | Xylanase/beta-glucanase OS=Ruminococcus flavefaciens OX=1265 GN=xynD PE=3 SV=2 |
| P29126 | 7.65e-81 | 1 | 252 | 1 | 245 | Bifunctional endo-1,4-beta-xylanase XylA OS=Ruminococcus flavefaciens OX=1265 GN=xynA PE=3 SV=1 |
| P17137 | 7.64e-71 | 43 | 248 | 72 | 260 | Endo-1,4-beta-xylanase OS=Clostridium saccharobutylicum OX=169679 GN=xynB PE=3 SV=1 |
| Q8GJ44 | 1.09e-68 | 43 | 264 | 44 | 248 | Endo-1,4-beta-xylanase A OS=Thermoclostridium stercorarium OX=1510 GN=xynA PE=1 SV=2 |
| P33558 | 2.35e-66 | 43 | 264 | 44 | 249 | Endo-1,4-beta-xylanase A OS=Thermoclostridium stercorarium OX=1510 GN=xynA PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
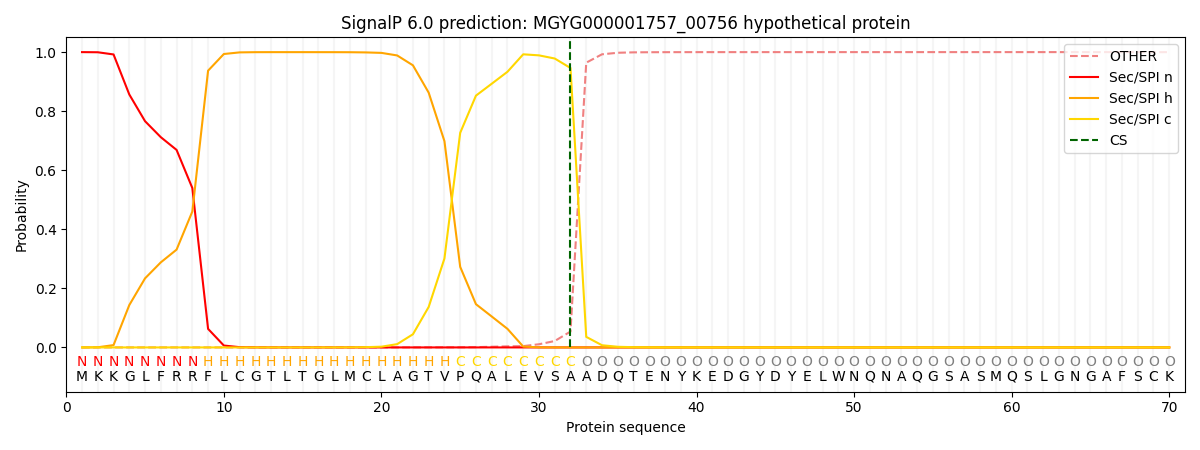
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000532 | 0.998361 | 0.000201 | 0.000431 | 0.000236 | 0.000193 |
