You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001770_00932
You are here: Home > Sequence: MGYG000001770_00932
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp900313215 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900313215 | |||||||||||
| CAZyme ID | MGYG000001770_00932 | |||||||||||
| CAZy Family | GH87 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 11520; End: 15563 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH87 | 44 | 602 | 8e-155 | 0.8458961474036851 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd14490 | CBM6-CBM35-CBM36_like_1 | 1.02e-40 | 45 | 209 | 1 | 156 | uncharacterized members of the carbohydrate binding module 6 (CBM6) and CBM35_like superfamily. Carbohydrate binding module family 6 (CBM6, family 6 CBM), also known as cellulose binding domain family VI (CBD VI), and related CBMs (CBM35 and CBM36). These are non-catalytic carbohydrate binding domains found in a range of enzymes that display activities against a diverse range of carbohydrate targets, including mannan, xylan, beta-glucans, cellulose, agarose, and arabinans. These domains facilitate the strong binding of the appended catalytic modules to their dedicated, insoluble substrates. Many of these CBMs are associated with glycoside hydrolase (GH) domains. CBM6 is an unusual CBM as it represents a chimera of two distinct binding sites with different modes of binding: binding site I within the loop regions and binding site II on the concave face of the beta-sandwich fold. CBM36s are calcium-dependent xylan binding domains. CBM35s display conserved specificity through extensive sequence similarity, but divergent function through their appended catalytic modules. |
| pfam13306 | LRR_5 | 9.05e-21 | 1008 | 1120 | 4 | 114 | Leucine rich repeats (6 copies). This family includes a number of leucine rich repeats. This family contains a large number of BSPA-like surface antigens from Trichomonas vaginalis. |
| pfam13306 | LRR_5 | 1.12e-20 | 1008 | 1108 | 26 | 127 | Leucine rich repeats (6 copies). This family includes a number of leucine rich repeats. This family contains a large number of BSPA-like surface antigens from Trichomonas vaginalis. |
| sd00036 | LRR_3 | 1.08e-19 | 1008 | 1120 | 6 | 119 | leucine-rich repeats. A leucine-rich repeat (LRR) is a structural protein motif of 20-30 amino acids that is unusually rich in the hydrophobic amino acid leucine. The conserved eleven-residue sequence motif (LxxLxLxxN/CxL) within the LRRs corresponds to the beta-strand and adjacent loop regions, whereas the remaining parts of the repeats are variable. LRRs fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Leucine-rich repeats are usually involved in protein-protein interactions. |
| sd00036 | LRR_3 | 6.83e-19 | 1011 | 1112 | 32 | 136 | leucine-rich repeats. A leucine-rich repeat (LRR) is a structural protein motif of 20-30 amino acids that is unusually rich in the hydrophobic amino acid leucine. The conserved eleven-residue sequence motif (LxxLxLxxN/CxL) within the LRRs corresponds to the beta-strand and adjacent loop regions, whereas the remaining parts of the repeats are variable. LRRs fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Leucine-rich repeats are usually involved in protein-protein interactions. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUB45559.1 | 0.0 | 11 | 993 | 8 | 964 |
| ANR73273.1 | 0.0 | 11 | 993 | 7 | 963 |
| BBA29482.1 | 0.0 | 11 | 954 | 7 | 939 |
| AUI55285.1 | 0.0 | 11 | 993 | 8 | 964 |
| QUB58650.1 | 0.0 | 11 | 984 | 8 | 955 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5ZRU_A | 1.31e-157 | 45 | 561 | 13 | 527 | ChainA, Alpha-1,3-glucanase [Niallia circulans],5ZRU_C Chain C, Alpha-1,3-glucanase [Niallia circulans] |
| 7C7D_A | 8.27e-32 | 44 | 543 | 53 | 507 | Crystalstructure of the catalytic unit of thermostable GH87 alpha-1,3-glucanase from Streptomyces thermodiastaticus strain HF3-3 [Streptomyces thermodiastaticus],7C7D_B Crystal structure of the catalytic unit of thermostable GH87 alpha-1,3-glucanase from Streptomyces thermodiastaticus strain HF3-3 [Streptomyces thermodiastaticus] |
| 6K0M_A | 4.29e-20 | 45 | 487 | 14 | 423 | Catalyticdomain of GH87 alpha-1,3-glucanase from Paenibacillus glycanilyticus FH11 [Paenibacillus glycanilyticus],6K0N_A Catalytic domain of GH87 alpha-1,3-glucanase in complex with nigerose [Paenibacillus glycanilyticus] |
| 6K0P_A | 4.03e-19 | 45 | 487 | 14 | 423 | Catalyticdomain of GH87 alpha-1,3-glucanase D1045A in complex with nigerose [Paenibacillus glycanilyticus] |
| 6K0S_A | 4.03e-19 | 45 | 487 | 14 | 423 | Catalyticdomain of GH87 alpha-1,3-glucanase D1069A in complex with nigerose [Paenibacillus glycanilyticus],6K0V_A Catalytic domain of GH87 alpha-1,3-glucanase D1069A in complex with tetrasaccharides [Paenibacillus glycanilyticus],6K0V_B Catalytic domain of GH87 alpha-1,3-glucanase D1069A in complex with tetrasaccharides [Paenibacillus glycanilyticus],6K0V_C Catalytic domain of GH87 alpha-1,3-glucanase D1069A in complex with tetrasaccharides [Paenibacillus glycanilyticus],6K0V_D Catalytic domain of GH87 alpha-1,3-glucanase D1069A in complex with tetrasaccharides [Paenibacillus glycanilyticus] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
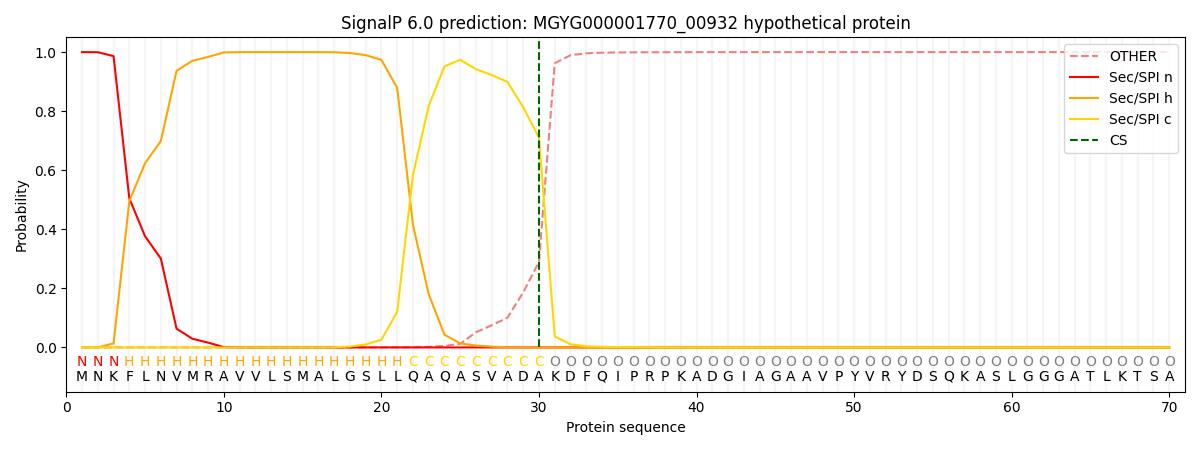
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000341 | 0.998868 | 0.000246 | 0.000186 | 0.000179 | 0.000158 |
