You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001783_00055
You are here: Home > Sequence: MGYG000001783_00055
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phocaeicola sp900551065 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Phocaeicola; Phocaeicola sp900551065 | |||||||||||
| CAZyme ID | MGYG000001783_00055 | |||||||||||
| CAZy Family | GH95 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 69700; End: 72192 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH95 | 50 | 818 | 3.1e-268 | 0.9903047091412742 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam14498 | Glyco_hyd_65N_2 | 3.68e-71 | 55 | 304 | 1 | 233 | Glycosyl hydrolase family 65, N-terminal domain. This domain represents a domain found to the N-terminus of the glycosyl hydrolase 65 family catalytic domain. |
| pfam04685 | DUF608 | 5.77e-04 | 512 | 613 | 108 | 201 | Glycosyl-hydrolase family 116, catalytic region. This represents a family of archaeal, bacterial and eukaryotic glycosyl hydrolases, that belong to superfamily GH116. The primary catabolic pathway for glucosylceramide is catalysis by the lysosomal enzyme glucocerebrosidase. In higher eukaryotes, glucosylceramide is the precursor of glycosphingolipids, a complex group of ubiquitous membrane lipids. Mutations in the human protein cause motor-neurone defects in hereditary spastic paraplegia. The catalytic nucleophile, identified in UniProtKB:Q97YG8_SULSO, is a glutamine-335, with the likely acid/base at Asp-442 and the aspartates at Asp-406 and Asp-458 residues also playing a role in the catalysis of glucosides and xylosides that are beta-bound to hydrophobic groups. The family is defined as GH116, which presently includes enzymes with beta-glucosidase, EC:3.2.1.21, beta-xylosidase, EC:3.2.1.37, and glucocerebrosidase EC:3.2.1.45 activity. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJR97976.1 | 0.0 | 53 | 830 | 1 | 778 |
| QCD34846.1 | 0.0 | 42 | 829 | 16 | 804 |
| QCP71443.1 | 0.0 | 51 | 829 | 24 | 802 |
| QCD40341.1 | 0.0 | 51 | 829 | 24 | 802 |
| QCD41177.1 | 0.0 | 37 | 830 | 9 | 802 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2RDY_A | 1.47e-185 | 53 | 804 | 3 | 746 | ChainA, BH0842 protein [Halalkalibacterium halodurans C-125],2RDY_B Chain B, BH0842 protein [Halalkalibacterium halodurans C-125] |
| 7KMQ_A | 8.77e-163 | 39 | 816 | 29 | 763 | ChainA, Glyco_hyd_65N_2 domain-containing protein [Xanthomonas citri pv. citri str. 306],7KMQ_B Chain B, Glyco_hyd_65N_2 domain-containing protein [Xanthomonas citri pv. citri str. 306] |
| 4UFC_A | 1.15e-156 | 46 | 810 | 15 | 741 | Crystalstructure of the GH95 enzyme BACOVA_03438 [Bacteroides ovatus],4UFC_B Crystal structure of the GH95 enzyme BACOVA_03438 [Bacteroides ovatus] |
| 2EAB_A | 1.05e-90 | 65 | 805 | 49 | 844 | Crystalstructure of 1,2-a-L-fucosidase from Bifidobacterium bifidum (apo form) [Bifidobacterium bifidum],2EAB_B Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum (apo form) [Bifidobacterium bifidum],2EAC_A Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum in complex with deoxyfuconojirimycin [Bifidobacterium bifidum],2EAC_B Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum in complex with deoxyfuconojirimycin [Bifidobacterium bifidum] |
| 2EAD_A | 7.44e-90 | 65 | 805 | 49 | 844 | ChainA, Alpha-fucosidase [Bifidobacterium bifidum],2EAD_B Chain B, Alpha-fucosidase [Bifidobacterium bifidum] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8L7W8 | 9.47e-165 | 53 | 814 | 52 | 816 | Alpha-L-fucosidase 2 OS=Arabidopsis thaliana OX=3702 GN=FUC95A PE=1 SV=1 |
| A2R797 | 7.39e-93 | 38 | 795 | 11 | 761 | Probable alpha-fucosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=afcA PE=3 SV=1 |
| Q5AU81 | 7.09e-86 | 69 | 815 | 45 | 801 | Alpha-fucosidase A OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=afcA PE=1 SV=1 |
| Q2USL3 | 7.89e-55 | 46 | 790 | 11 | 694 | Probable alpha-fucosidase A OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=afcA PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
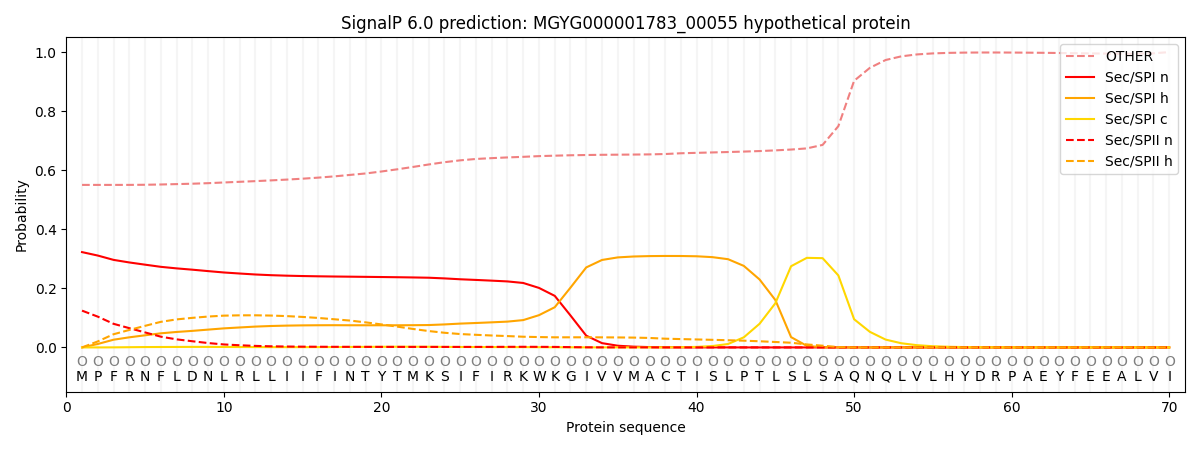
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.559030 | 0.306052 | 0.130385 | 0.001835 | 0.001702 | 0.000998 |
