You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001806_01937
You are here: Home > Sequence: MGYG000001806_01937
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp900553965 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900553965 | |||||||||||
| CAZyme ID | MGYG000001806_01937 | |||||||||||
| CAZy Family | GH13 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8490; End: 10484 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 58 | 340 | 1.8e-47 | 0.9688581314878892 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd11314 | AmyAc_arch_bac_plant_AmyA | 1.30e-108 | 29 | 367 | 1 | 300 | Alpha amylase catalytic domain found in archaeal, bacterial, and plant Alpha-amylases (also called 1,4-alpha-D-glucan-4-glucanohydrolase). AmyA (EC 3.2.1.1) catalyzes the hydrolysis of alpha-(1,4) glycosidic linkages of glycogen, starch, related polysaccharides, and some oligosaccharides. This group includes AmyA from bacteria, archaea, water fleas, and plants. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| PRK09441 | PRK09441 | 4.14e-42 | 27 | 359 | 3 | 392 | cytoplasmic alpha-amylase; Reviewed |
| PLN02361 | PLN02361 | 2.72e-40 | 29 | 359 | 13 | 343 | alpha-amylase |
| PLN02784 | PLN02784 | 1.24e-36 | 29 | 363 | 504 | 837 | alpha-amylase |
| cd11318 | AmyAc_bac_fung_AmyA | 1.55e-33 | 28 | 360 | 2 | 391 | Alpha amylase catalytic domain found in bacterial and fungal Alpha amylases (also called 1,4-alpha-D-glucan-4-glucanohydrolase). AmyA (EC 3.2.1.1) catalyzes the hydrolysis of alpha-(1,4) glycosidic linkages of glycogen, starch, related polysaccharides, and some oligosaccharides. This group includes bacterial and fungal proteins. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QNT66936.1 | 2.37e-271 | 1 | 664 | 1 | 672 |
| QUB75387.1 | 1.99e-246 | 1 | 664 | 1 | 675 |
| QUB80123.1 | 4.42e-240 | 1 | 664 | 1 | 675 |
| QUB62461.1 | 6.98e-240 | 19 | 664 | 2 | 658 |
| AKU69270.1 | 2.44e-239 | 1 | 664 | 1 | 674 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1AMY_A | 4.59e-31 | 29 | 367 | 2 | 350 | CrystalAnd Molecular Structure Of Barley Alpha-Amylase [Hordeum vulgare],1AVA_A Amy2BASI PROTEIN-Protein Complex From Barley Seed [Hordeum vulgare],1AVA_B Amy2BASI PROTEIN-Protein Complex From Barley Seed [Hordeum vulgare],1BG9_A Barley Alpha-Amylase With Substrate Analogue Acarbose [Hordeum vulgare] |
| 3BSH_A | 7.39e-31 | 29 | 427 | 3 | 409 | ChainA, Alpha-amylase type A isozyme [Hordeum vulgare] |
| 3BSG_A | 1.35e-30 | 29 | 427 | 3 | 409 | ChainA, Alpha-amylase type A isozyme [Hordeum vulgare] |
| 1HT6_A | 1.59e-30 | 29 | 367 | 3 | 352 | CrystalStructure At 1.5a Resolution Of The Barley Alpha- Amylase Isozyme 1 [Hordeum vulgare],1P6W_A Crystal structure of barley alpha-amylase isozyme 1 (AMY1) in complex with the substrate analogue, methyl 4I,4II,4III-tri-thiomaltotetraoside (thio-DP4) [Hordeum vulgare],1RPK_A Crystal structure of barley alpha-amylase isozyme 1 (amy1) in complex with acarbose [Hordeum vulgare] |
| 2QPS_A | 1.59e-30 | 29 | 367 | 3 | 352 | ChainA, Alpha-amylase type A isozyme [Hordeum vulgare] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8LFG1 | 1.68e-34 | 29 | 417 | 27 | 409 | Probable alpha-amylase 2 OS=Arabidopsis thaliana OX=3702 GN=AMY2 PE=2 SV=1 |
| P27933 | 2.48e-34 | 1 | 417 | 1 | 421 | Alpha-amylase isozyme 3D OS=Oryza sativa subsp. japonica OX=39947 GN=AMY1.3 PE=2 SV=2 |
| P17859 | 3.57e-34 | 29 | 417 | 25 | 418 | Alpha-amylase OS=Vigna mungo OX=3915 GN=AMY1.1 PE=2 SV=1 |
| P27932 | 9.01e-34 | 29 | 416 | 30 | 423 | Alpha-amylase isozyme 3A OS=Oryza sativa subsp. japonica OX=39947 GN=AMY1.2 PE=2 SV=2 |
| P27939 | 2.46e-32 | 29 | 363 | 28 | 370 | Alpha-amylase isozyme 3C OS=Oryza sativa subsp. japonica OX=39947 GN=AMY1.7 PE=2 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
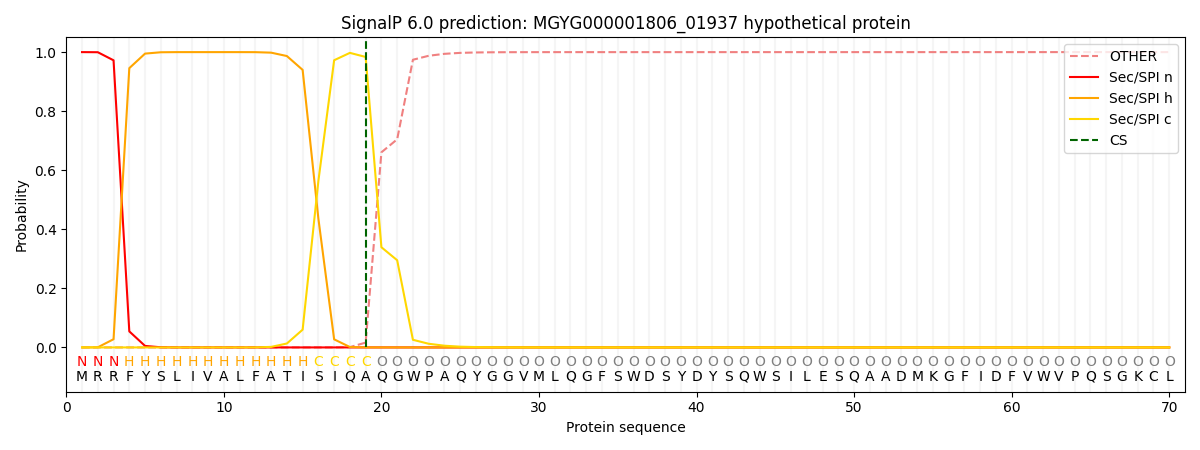
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000384 | 0.998793 | 0.000168 | 0.000225 | 0.000202 | 0.000187 |
