You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001851_00329
You are here: Home > Sequence: MGYG000001851_00329
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
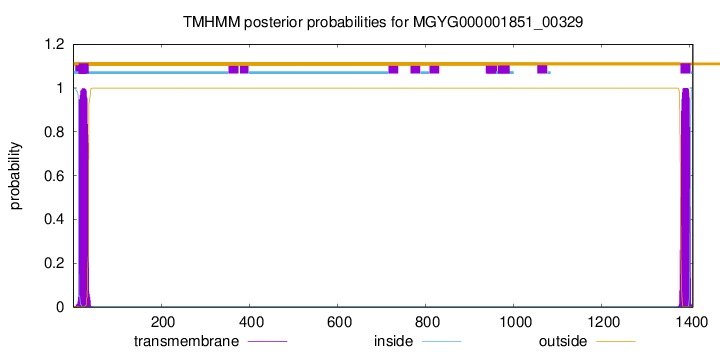
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia_A; Christensenellales; CAG-552; UMGS1880; | |||||||||||
| CAZyme ID | MGYG000001851_00329 | |||||||||||
| CAZy Family | GH3 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 6034; End: 10257 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH3 | 822 | 1055 | 2.2e-39 | 0.9675925925925926 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| PRK15098 | PRK15098 | 2.21e-13 | 445 | 546 | 632 | 730 | beta-glucosidase BglX. |
| pfam01915 | Glyco_hydro_3_C | 6.98e-13 | 76 | 342 | 2 | 184 | Glycosyl hydrolase family 3 C-terminal domain. This domain is involved in catalysis and may be involved in binding beta-glucan. This domain is found associated with pfam00933. |
| pfam14310 | Fn3-like | 1.81e-12 | 498 | 577 | 1 | 71 | Fibronectin type III-like domain. This domain has a fibronectin type III-like structure. It is often found in association with pfam00933 and pfam01915. Its function is unknown. |
| pfam01391 | Collagen | 6.70e-11 | 1306 | 1371 | 1 | 55 | Collagen triple helix repeat (20 copies). Members of this family belong to the collagen superfamily. Collagens are generally extracellular structural proteins involved in formation of connective tissue structure. The alignment contains 20 copies of the G-X-Y repeat that forms a triple helix. The first position of the repeat is glycine, the second and third positions can be any residue but are frequently proline and hydroxy-proline. Collagens are post translationally modified by proline hydroxylase to form the hydroxy-proline residues. Defective hydroxylation is the cause of scurvy. Some members of the collagen superfamily are not involved in connective tissue structure but share the same triple helical structure. The family includes bacterial collagen-like triple-helix repeat proteins. |
| pfam00933 | Glyco_hydro_3 | 1.37e-07 | 859 | 1057 | 86 | 283 | Glycosyl hydrolase family 3 N terminal domain. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| VEU80232.1 | 3.43e-166 | 48 | 1119 | 65 | 917 |
| VEU80230.1 | 9.95e-158 | 49 | 1213 | 29 | 996 |
| QFJ55185.1 | 6.36e-154 | 46 | 1058 | 58 | 918 |
| SQH20118.1 | 6.82e-138 | 43 | 1120 | 54 | 922 |
| ASE08187.2 | 6.82e-138 | 43 | 1120 | 54 | 922 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5WUG_A | 9.12e-54 | 60 | 1055 | 33 | 776 | Expression,characterization and crystal structure of a novel beta-glucosidase from Paenibacillus barengoltzii [Paenibacillus barengoltzii],5WVP_A Expression, characterization and crystal structure of a novel beta-glucosidase from Paenibacillus barengoltzii [Paenibacillus barengoltzii] |
| 2X42_A | 2.33e-26 | 73 | 577 | 334 | 702 | Structureof beta-glucosidase 3B from Thermotoga neapolitana in complex with alpha-D-glucose [Thermotoga neapolitana DSM 4359] |
| 2X40_A | 2.33e-26 | 73 | 577 | 334 | 702 | Structureof beta-glucosidase 3B from Thermotoga neapolitana in complex with glycerol [Thermotoga neapolitana DSM 4359],2X41_A Structure of beta-glucosidase 3B from Thermotoga neapolitana in complex with glucose [Thermotoga neapolitana DSM 4359] |
| 7MS2_A | 1.77e-22 | 261 | 573 | 418 | 653 | ChainA, Thermostable beta-glucosidase B [Acetivibrio thermocellus AD2],7MS2_B Chain B, Thermostable beta-glucosidase B [Acetivibrio thermocellus AD2] |
| 5WAB_A | 2.09e-21 | 796 | 1047 | 9 | 251 | CrystalStructure of Bifidobacterium adolescentis GH3 beta-glucosidase [Bifidobacterium adolescentis ATCC 15703],5WAB_B Crystal Structure of Bifidobacterium adolescentis GH3 beta-glucosidase [Bifidobacterium adolescentis ATCC 15703],5WAB_C Crystal Structure of Bifidobacterium adolescentis GH3 beta-glucosidase [Bifidobacterium adolescentis ATCC 15703],5WAB_D Crystal Structure of Bifidobacterium adolescentis GH3 beta-glucosidase [Bifidobacterium adolescentis ATCC 15703] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P16084 | 1.86e-61 | 189 | 1034 | 130 | 769 | Beta-glucosidase A OS=Butyrivibrio fibrisolvens OX=831 GN=bglA PE=3 SV=1 |
| P15885 | 1.36e-34 | 271 | 1096 | 151 | 752 | Beta-glucosidase OS=Ruminococcus albus OX=1264 PE=3 SV=1 |
| P27034 | 5.92e-24 | 793 | 1040 | 3 | 228 | Beta-glucosidase OS=Rhizobium radiobacter OX=358 GN=cbg-1 PE=3 SV=1 |
| P14002 | 9.71e-22 | 261 | 573 | 418 | 653 | Thermostable beta-glucosidase B OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=bglB PE=1 SV=2 |
| Q2U9M7 | 1.10e-21 | 865 | 1047 | 67 | 242 | Probable beta-glucosidase H OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=bglH PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.074769 | 0.922173 | 0.002203 | 0.000330 | 0.000241 | 0.000252 |