You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001906_00067
You are here: Home > Sequence: MGYG000001906_00067
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
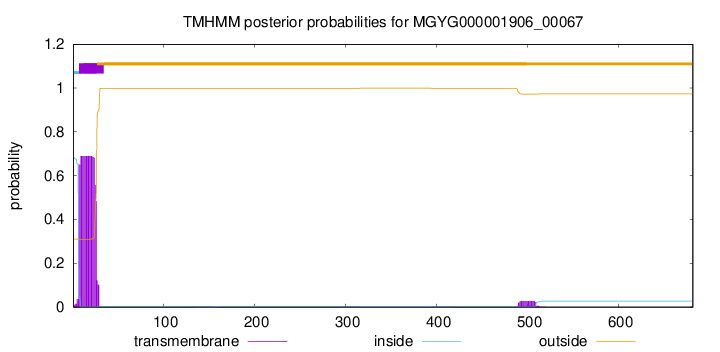
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Cyanobacteria; Vampirovibrionia; Gastranaerophilales; Gastranaerophilaceae; CAG-484; | |||||||||||
| CAZyme ID | MGYG000001906_00067 | |||||||||||
| CAZy Family | GH13 | |||||||||||
| CAZyme Description | Trehalose synthase/amylase TreS | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 70481; End: 72529 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 126 | 513 | 8.5e-121 | 0.9830028328611898 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd11334 | AmyAc_TreS | 4.31e-113 | 101 | 512 | 1 | 373 | Alpha amylase catalytic domain found in Trehalose synthetase. Trehalose synthetase (TreS) catalyzes the reversible interconversion of trehalose and maltose. The enzyme catalyzes the reaction in both directions, but the preferred substrate is maltose. Glucose is formed as a by-product of this reaction. It is believed that the catalytic mechanism may involve the cutting of the incoming disaccharide and transfer of a glucose to an enzyme-bound glucose. This enzyme also catalyzes production of a glucosamine disaccharide from maltose and glucosamine. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| cd11324 | AmyAc_Amylosucrase | 2.52e-84 | 81 | 587 | 43 | 535 | Alpha amylase catalytic domain found in Amylosucrase. Amylosucrase is a glucosyltransferase that catalyzes the transfer of a D-glucopyranosyl moiety from sucrose onto an acceptor molecule. When the acceptor is another saccharide, only alpha-1,4 linkages are produced. Unlike most amylopolysaccharide synthases, it does not require any alpha-D-glucosyl nucleoside diphosphate substrate. In the presence of glycogen it catalyzes the transfer of a D-glucose moiety onto a glycogen branch, but in its absence, it hydrolyzes sucrose and synthesizes polymers, smaller maltosaccharides, and sucrose isoforms. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| COG0366 | AmyA | 1.06e-60 | 106 | 645 | 2 | 505 | Glycosidase [Carbohydrate transport and metabolism]. |
| cd11316 | AmyAc_bac2_AmyA | 2.14e-52 | 117 | 591 | 8 | 403 | Alpha amylase catalytic domain found in bacterial Alpha-amylases (also called 1,4-alpha-D-glucan-4-glucanohydrolase). AmyA (EC 3.2.1.1) catalyzes the hydrolysis of alpha-(1,4) glycosidic linkages of glycogen, starch, related polysaccharides, and some oligosaccharides. This group includes Chloroflexi, Dictyoglomi, and Fusobacteria. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| cd11356 | AmyAc_Sucrose_phosphorylase-like_1 | 2.44e-49 | 101 | 586 | 1 | 440 | Alpha amylase catalytic domain found in sucrose phosphorylase-like proteins (also called sucrose glucosyltransferase, disaccharide glucosyltransferase, and sucrose-phosphate alpha-D glucosyltransferase). Sucrose phosphorylase is a bacterial enzyme that catalyzes the phosphorolysis of sucrose to yield glucose-1-phosphate and fructose. These enzymes do not have the conserved calcium ion present in other alpha amylase family enzymes. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AOR37731.1 | 2.86e-269 | 30 | 680 | 28 | 677 |
| AKB34836.1 | 1.39e-248 | 51 | 678 | 24 | 648 |
| QCR16547.1 | 1.18e-222 | 47 | 678 | 5 | 667 |
| BBL63642.1 | 1.18e-222 | 47 | 678 | 5 | 667 |
| AKB40115.1 | 1.18e-222 | 47 | 678 | 5 | 667 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5X7U_A | 1.15e-91 | 95 | 632 | 1 | 502 | Trehalosesynthase from Thermobaculum terrenum [Thermobaculum terrenum ATCC BAA-798] |
| 5H2T_A | 7.98e-86 | 101 | 632 | 22 | 521 | Structureof trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_B Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_C Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_D Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_E Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_F Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_G Structure of trehalose synthase [Thermomonospora curvata DSM 43183],5H2T_H Structure of trehalose synthase [Thermomonospora curvata DSM 43183] |
| 4WF7_A | 1.65e-82 | 100 | 682 | 9 | 555 | Crystalstructures of trehalose synthase from Deinococcus radiodurans reveal that a closed conformation is involved in the intramolecular isomerization catalysis [Deinococcus radiodurans R1],4WF7_B Crystal structures of trehalose synthase from Deinococcus radiodurans reveal that a closed conformation is involved in the intramolecular isomerization catalysis [Deinococcus radiodurans R1],4WF7_C Crystal structures of trehalose synthase from Deinococcus radiodurans reveal that a closed conformation is involved in the intramolecular isomerization catalysis [Deinococcus radiodurans R1],4WF7_D Crystal structures of trehalose synthase from Deinococcus radiodurans reveal that a closed conformation is involved in the intramolecular isomerization catalysis [Deinococcus radiodurans R1] |
| 5YKB_A | 3.22e-82 | 100 | 682 | 9 | 555 | TheN253F mutant structure of trehalose synthase from Deinococcus radiodurans reveals an open active-site conformation [Deinococcus radiodurans R1],5YKB_B The N253F mutant structure of trehalose synthase from Deinococcus radiodurans reveals an open active-site conformation [Deinococcus radiodurans R1],5YKB_C The N253F mutant structure of trehalose synthase from Deinococcus radiodurans reveals an open active-site conformation [Deinococcus radiodurans R1],5YKB_D The N253F mutant structure of trehalose synthase from Deinococcus radiodurans reveals an open active-site conformation [Deinococcus radiodurans R1] |
| 5GTW_A | 4.50e-82 | 100 | 682 | 9 | 555 | TheN253R mutant structures of trehalose synthase from Deinococcus radiodurans display two different active-site conformations [Deinococcus radiodurans R1],5GTW_B The N253R mutant structures of trehalose synthase from Deinococcus radiodurans display two different active-site conformations [Deinococcus radiodurans R1],5GTW_C The N253R mutant structures of trehalose synthase from Deinococcus radiodurans display two different active-site conformations [Deinococcus radiodurans R1],5GTW_D The N253R mutant structures of trehalose synthase from Deinococcus radiodurans display two different active-site conformations [Deinococcus radiodurans R1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P9WQ18 | 2.80e-79 | 101 | 647 | 43 | 557 | Trehalose synthase/amylase TreS OS=Mycobacterium tuberculosis (strain CDC 1551 / Oshkosh) OX=83331 GN=treS PE=3 SV=1 |
| P9WQ19 | 2.80e-79 | 101 | 647 | 43 | 557 | Trehalose synthase/amylase TreS OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=treS PE=1 SV=1 |
| A0R6E0 | 2.38e-78 | 99 | 640 | 33 | 548 | Trehalose synthase/amylase TreS OS=Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) OX=246196 GN=treS PE=1 SV=1 |
| P72235 | 8.08e-77 | 95 | 632 | 9 | 523 | Trehalose synthase OS=Pimelobacter sp. (strain R48) OX=51662 GN=treS PE=3 SV=1 |
| O06458 | 7.17e-69 | 101 | 635 | 5 | 500 | Trehalose synthase OS=Thermus thermophilus OX=274 GN=treS PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
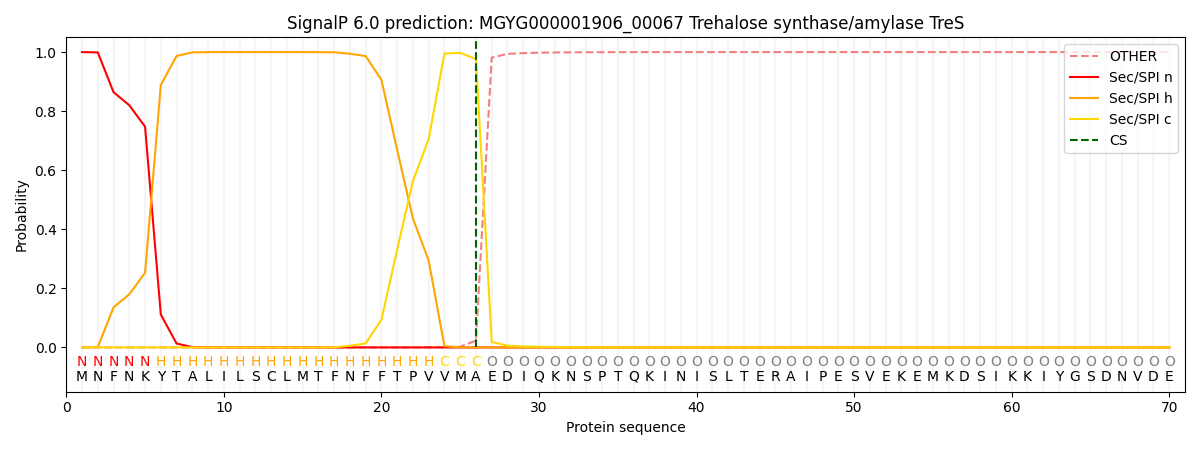
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000246 | 0.999111 | 0.000176 | 0.000156 | 0.000154 | 0.000142 |