You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001929_01070
You are here: Home > Sequence: MGYG000001929_01070
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
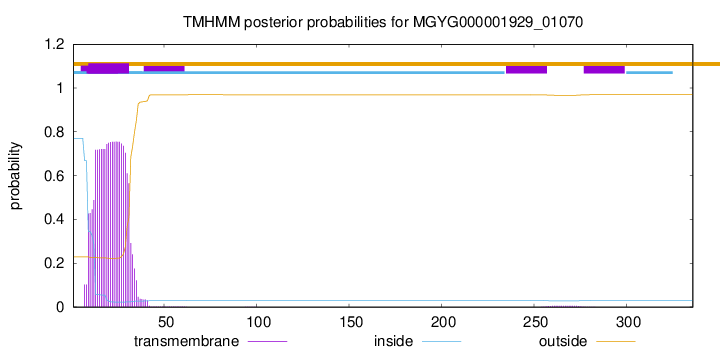
TMHMM annotations
Basic Information help
| Species | Paramuribaculum sp900752995 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; Paramuribaculum; Paramuribaculum sp900752995 | |||||||||||
| CAZyme ID | MGYG000001929_01070 | |||||||||||
| CAZy Family | GT9 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 58266; End: 59276 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT9 | 74 | 313 | 1.6e-31 | 0.9644444444444444 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd03789 | GT9_LPS_heptosyltransferase | 2.85e-47 | 7 | 332 | 1 | 277 | lipopolysaccharide heptosyltransferase and similar proteins. Lipopolysaccharide heptosyltransferase (2.4.99.B6) is involved in the biosynthesis of lipooligosaccharide (LOS). Lipopolysaccharide (LPS) is a major component of the outer membrane of gram-negative bacteria. LPS heptosyltransferase transfers heptose molecules from ADP-heptose to 3-deoxy-D-manno-octulosonic acid (KDO), a part of the inner core component of LPS. This family also contains lipopolysaccharide 1,2-N-acetylglucosaminetransferase EC 2.4.1.56 and belongs to the GT-B structural superfamily of glycoslytransferases, which have characteristic N- and C-terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. |
| COG0859 | RfaF | 7.24e-38 | 6 | 334 | 2 | 330 | ADP-heptose:LPS heptosyltransferase [Cell wall/membrane/envelope biogenesis]. |
| pfam01075 | Glyco_transf_9 | 1.17e-20 | 73 | 308 | 1 | 242 | Glycosyltransferase family 9 (heptosyltransferase). Members of this family belong to glycosyltransferase family 9. Lipopolysaccharide is a major component of the outer leaflet of the outer membrane in Gram-negative bacteria. It is composed of three domains; lipid A, Core oligosaccharide and the O-antigen. All of these enzymes transfer heptose to the lipopolysaccharide core. |
| PRK10916 | PRK10916 | 3.95e-11 | 160 | 294 | 171 | 308 | ADP-heptose--LPS heptosyltransferase RfaF. |
| PRK10422 | PRK10422 | 1.67e-08 | 1 | 274 | 1 | 291 | lipopolysaccharide core biosynthesis protein; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QCD43584.1 | 1.34e-119 | 6 | 335 | 4 | 333 |
| QUB41015.1 | 4.11e-83 | 4 | 334 | 5 | 331 |
| ATV54740.1 | 2.26e-70 | 4 | 330 | 2 | 344 |
| BAU17893.1 | 6.37e-70 | 4 | 330 | 2 | 344 |
| AVV53353.1 | 6.37e-70 | 9 | 334 | 5 | 341 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1PSW_A | 9.25e-10 | 165 | 294 | 176 | 308 | Structureof E. coli ADP-heptose lps heptosyltransferase II [Escherichia coli] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P37421 | 1.18e-09 | 158 | 294 | 169 | 308 | ADP-heptose--LPS heptosyltransferase 2 OS=Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) OX=99287 GN=rfaF PE=3 SV=1 |
| P37692 | 3.78e-09 | 165 | 294 | 176 | 308 | ADP-heptose--LPS heptosyltransferase 2 OS=Escherichia coli (strain K12) OX=83333 GN=rfaF PE=1 SV=1 |
| P45042 | 1.61e-08 | 168 | 294 | 179 | 308 | ADP-heptose--LPS heptosyltransferase 2 OS=Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) OX=71421 GN=rfaF PE=3 SV=1 |
| Q57336 | 1.65e-07 | 2 | 276 | 5 | 286 | Lipopolysaccharide core heptosyltransferase OpsX OS=Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) OX=71421 GN=opsX PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
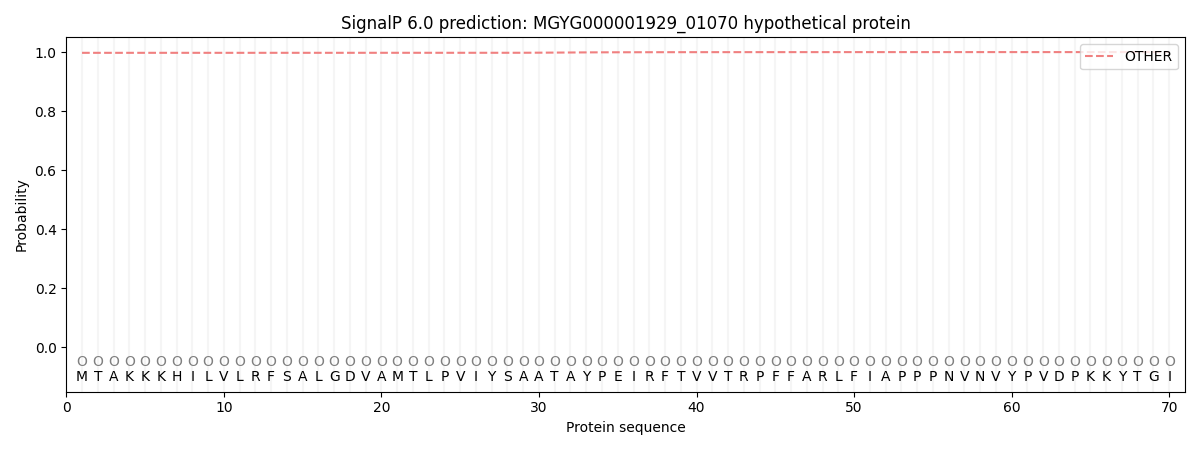
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.997727 | 0.002248 | 0.000036 | 0.000007 | 0.000003 | 0.000005 |