You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001977_01071
You are here: Home > Sequence: MGYG000001977_01071
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; | |||||||||||
| CAZyme ID | MGYG000001977_01071 | |||||||||||
| CAZy Family | GH116 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 15472; End: 18036 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH116 | 414 | 782 | 1.2e-62 | 0.9862258953168044 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam04685 | DUF608 | 2.08e-69 | 410 | 782 | 1 | 362 | Glycosyl-hydrolase family 116, catalytic region. This represents a family of archaeal, bacterial and eukaryotic glycosyl hydrolases, that belong to superfamily GH116. The primary catabolic pathway for glucosylceramide is catalysis by the lysosomal enzyme glucocerebrosidase. In higher eukaryotes, glucosylceramide is the precursor of glycosphingolipids, a complex group of ubiquitous membrane lipids. Mutations in the human protein cause motor-neurone defects in hereditary spastic paraplegia. The catalytic nucleophile, identified in UniProtKB:Q97YG8_SULSO, is a glutamine-335, with the likely acid/base at Asp-442 and the aspartates at Asp-406 and Asp-458 residues also playing a role in the catalysis of glucosides and xylosides that are beta-bound to hydrophobic groups. The family is defined as GH116, which presently includes enzymes with beta-glucosidase, EC:3.2.1.21, beta-xylosidase, EC:3.2.1.37, and glucocerebrosidase EC:3.2.1.45 activity. |
| pfam12215 | Glyco_hydr_116N | 4.86e-45 | 43 | 368 | 1 | 309 | beta-glucosidase 2, glycosyl-hydrolase family 116 N-term. This domain is found in bacteria, archaea and eukaryotes. This domain is typically between 320 to 354 amino acids in length. This domain is found associated with pfam04685. It is found just after the extreme N-terminus. The N-terminal is thought to be the luminal domain while the C terminal is the cytosolic domain. The catalytic domain of GBA-2 is unknown. The primary catabolic pathway for glucosylceramide is catalysis by the lysosomal enzyme glucocerebrosidase. In higher eukaryotes, glucosylceramide is the precursor of glycosphingolipids, a complex group of ubiquitous membrane lipids. Mutations in the human protein cause motor-neurone defects in hereditary spastic paraplegia. The catalytic nucleophile, identified in UniProtKB:Q97YG8_SULSO, is a glutamine-335 in the downstream family pfam04685. |
| COG4354 | COG4354 | 1.18e-39 | 45 | 790 | 16 | 721 | Uncharacterized protein, contains GBA2_N and DUF608 domains [Function unknown]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT60790.1 | 0.0 | 1 | 851 | 1 | 851 |
| QQA29305.1 | 0.0 | 1 | 851 | 1 | 851 |
| QUT66528.1 | 0.0 | 1 | 851 | 1 | 851 |
| BBK87334.1 | 0.0 | 1 | 851 | 8 | 858 |
| QQA29312.1 | 0.0 | 9 | 853 | 10 | 851 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7DKW_A | 1.72e-08 | 45 | 744 | 59 | 736 | ChainA, beta-glucosidase [Thermoanaerobacterium xylanolyticum LX-11],7DKW_B Chain B, beta-glucosidase [Thermoanaerobacterium xylanolyticum LX-11],7DKX_A Chain A, beta-glucosidase [Thermoanaerobacterium xylanolyticum LX-11],7DKY_A Chain A, beta-glucosidase [Thermoanaerobacterium xylanolyticum LX-11] |
| 5FJS_A | 2.96e-08 | 45 | 744 | 55 | 732 | Bacterialbeta-glucosidase reveals the structural and functional basis of genetic defects in human glucocerebrosidase 2 (GBA2) [Thermoanaerobacterium xylanolyticum LX-11],5FJS_B Bacterial beta-glucosidase reveals the structural and functional basis of genetic defects in human glucocerebrosidase 2 (GBA2) [Thermoanaerobacterium xylanolyticum LX-11],5O0S_A Crystal structure of txGH116 (beta-glucosidase from Thermoanaerobacterium xylolyticum) in complex with unreacted beta Cyclophellitol Cyclosulfate probe ME711 [Thermoanaerobacterium xylanolyticum LX-11] |
| 5BVU_A | 2.98e-08 | 45 | 744 | 59 | 736 | Crystalstructure of Thermoanaerobacterium xylolyticum GH116 beta-glucosidase [Thermoanaerobacterium xylanolyticum LX-11],5BX2_A Crystal structure of Thermoanaerobacterium xylanolyticum GH116 beta-glucosidase with 2-deoxy-2-fluoroglucoside [Thermoanaerobacterium xylanolyticum LX-11],5BX3_A Crystal structure of Thermoanaerobacterium xylanolyticum GH116 beta-glucosidase with deoxynojirimycin [Thermoanaerobacterium xylanolyticum LX-11],5BX4_A Crystal structure of Thermoanaerobacterium xylanolyticum GH116 beta-glucosidase with Glucoimidazole [Thermoanaerobacterium xylanolyticum LX-11],5BX5_A Crystal structure of Thermoanaerobacterium xylanolyticum GH116 beta-glucosidase with glucose [Thermoanaerobacterium xylanolyticum LX-11],5NCX_A Crystal structure of Thermoanaerobacterium xylolyticum GH116 beta-glucosidase with an covalent inhibitor [Thermoanaerobacterium xylanolyticum LX-11],5OST_A Beta-glucosidase from Thermoanaerobacterium xylolyticum GH116 in complex with Gluco-1H-imidazole [Thermoanaerobacterium xylanolyticum LX-11] |
| 5NPF_A | 2.98e-08 | 45 | 744 | 58 | 735 | Crystalstructure of txGH116 (beta-glucosidase from Thermoanaerobacterium xylolyticum) in complex with beta Cyclophellitol Cyclosulfate probe ME594 [Thermoanaerobacterium xylanolyticum] |
| 7W2V_A | 3.04e-08 | 45 | 744 | 102 | 779 | ChainA, Glucosylceramidase [Thermoanaerobacterium xylanolyticum LX-11] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q69ZF3 | 1.66e-09 | 45 | 752 | 144 | 839 | Non-lysosomal glucosylceramidase OS=Mus musculus OX=10090 GN=Gba2 PE=1 SV=2 |
| Q5M868 | 2.18e-09 | 20 | 752 | 116 | 839 | Non-lysosomal glucosylceramidase OS=Rattus norvegicus OX=10116 GN=Gba2 PE=2 SV=2 |
| Q9HCG7 | 3.78e-09 | 117 | 752 | 218 | 848 | Non-lysosomal glucosylceramidase OS=Homo sapiens OX=9606 GN=GBA2 PE=1 SV=2 |
| Q7KT91 | 5.83e-08 | 493 | 747 | 678 | 893 | Non-lysosomal glucosylceramidase OS=Drosophila melanogaster OX=7227 GN=CG33090 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
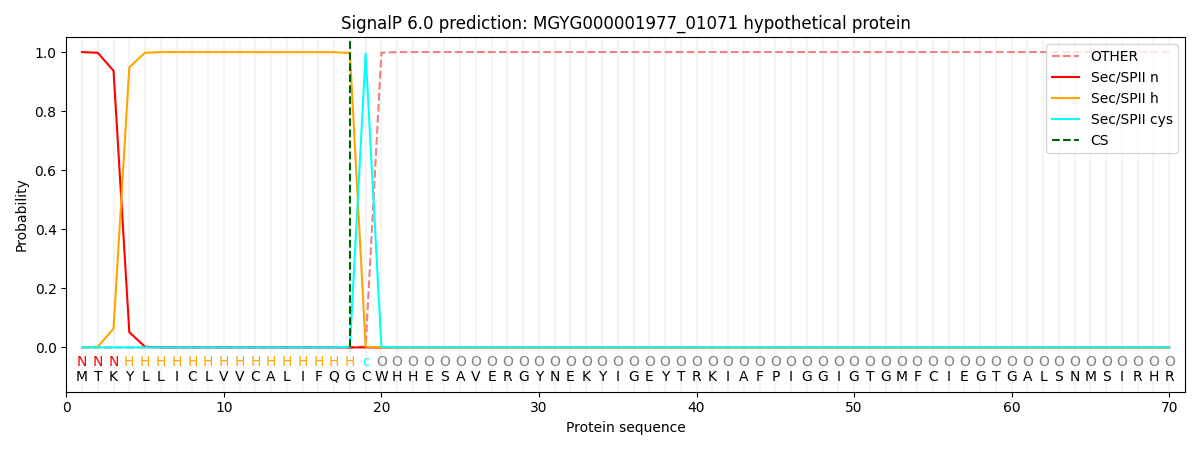
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000001 | 1.000048 | 0.000000 | 0.000000 | 0.000000 |
