You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001995_01889
You are here: Home > Sequence: MGYG000001995_01889
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parabacteroides sp900541965 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides sp900541965 | |||||||||||
| CAZyme ID | MGYG000001995_01889 | |||||||||||
| CAZy Family | GH38 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2760; End: 5459 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH38 | 54 | 325 | 1.8e-40 | 0.9405204460966543 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10791 | GH38N_AMII_like_1 | 1.52e-45 | 54 | 287 | 2 | 253 | N-terminal catalytic domain of mainly uncharacterized eukaryotic proteins similar to alpha-mannosidases; glycoside hydrolase family 38 (GH38). The subfamily of mainly uncharacterized eukaryotic proteins shows sequence homology with class II alpha-mannosidases (AlphaAMIIs). AlphaAMIIs possess a-1,3, a-1,6, and a-1,2 hydrolytic activity, and catalyze the degradation of N-linked oligosaccharides. The N-terminal catalytic domain of alphaMII adopts a structure consisting of parallel 7-stranded beta/alpha barrel. This subfamily belongs to the GH38 family of retaining glycosyl hydrolases, which employ a two-step mechanism involving the formation of a covalent glycosyl enzyme complex; two carboxylic acids positioned within the active site act in concert: one as a catalytic nucleophile and the other as a general acid/base catalyst. |
| PRK09819 | PRK09819 | 1.17e-32 | 86 | 880 | 37 | 852 | mannosylglycerate hydrolase. |
| COG0383 | AMS1 | 2.64e-32 | 53 | 899 | 199 | 943 | Alpha-mannosidase [Carbohydrate transport and metabolism]. |
| cd10786 | GH38N_AMII_like | 3.35e-32 | 53 | 288 | 1 | 251 | N-terminal catalytic domain of class II alpha-mannosidases and similar proteins; glycoside hydrolase family 38 (GH38). Alpha-mannosidases (EC 3.2.1.24) are extensively found in eukaryotes and play important roles in the processing of newly formed N-glycans and in degradation of mature glycoproteins. A deficiency of this enzyme causes the lysosomal storage disease alpha-mannosidosis. Many bacterial and archaeal species also possess putative alpha-mannosidases, but their activity and specificity is largely unknown. Based on different functional characteristics and sequence homology, alpha-mannosidases have been organized into two classes (class I, belonging to glycoside hydrolase family 47, and class II, belonging to glycoside hydrolase family 38). Members of this family corresponds to class II alpha-mannosidases (alphaMII), which contain intermediate Golgi alpha-mannosidases II, acidic lysosomal alpha-mannosidases, animal sperm and epididymal alpha -mannosidases, neutral ER/cytosolic alpha-mannosidases, and some putative prokaryotic alpha-mannosidases. AlphaMII possess a-1,3, a-1,6, and a-1,2 hydrolytic activity, and catalyzes the degradation of N-linked oligosaccharides. The N-terminal catalytic domain of alphaMII adopts a structure consisting of parallel 7-stranded beta/alpha barrel. Members in this family are retaining glycosyl hydrolases of family GH38 that employs a two-step mechanism involving the formation of a covalent glycosyl enzyme complex. Two carboxylic acids positioned within the active site act in concert: one as a catalytic nucleophile and the other as a general acid/base catalyst. |
| pfam01074 | Glyco_hydro_38 | 5.13e-23 | 60 | 324 | 8 | 259 | Glycosyl hydrolases family 38 N-terminal domain. Glycosyl hydrolases are key enzymes of carbohydrate metabolism. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT96672.1 | 0.0 | 5 | 898 | 1 | 892 |
| AST52233.1 | 0.0 | 2 | 898 | 11 | 905 |
| APY11549.1 | 2.40e-229 | 36 | 899 | 311 | 1164 |
| QCX40403.1 | 6.33e-227 | 36 | 899 | 346 | 1201 |
| EAR02398.1 | 6.43e-225 | 36 | 897 | 330 | 1180 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6LZ1_A | 1.55e-10 | 34 | 899 | 249 | 1077 | Structureof S.pombe alpha-mannosidase Ams1 [Schizosaccharomyces pombe 972h-],6LZ1_B Structure of S.pombe alpha-mannosidase Ams1 [Schizosaccharomyces pombe 972h-],6LZ1_C Structure of S.pombe alpha-mannosidase Ams1 [Schizosaccharomyces pombe 972h-],6LZ1_D Structure of S.pombe alpha-mannosidase Ams1 [Schizosaccharomyces pombe 972h-] |
| 7DD9_A | 1.69e-10 | 34 | 899 | 249 | 1077 | ChainA, Alpha-mannosidase,ZZ-type zinc finger-containing protein P35G2.11c,Maltose/maltodextrin-binding periplasmic protein [synthetic construct],7DD9_C Chain C, Alpha-mannosidase,ZZ-type zinc finger-containing protein P35G2.11c,Maltose/maltodextrin-binding periplasmic protein [synthetic construct],7DD9_E Chain E, Alpha-mannosidase,ZZ-type zinc finger-containing protein P35G2.11c,Maltose/maltodextrin-binding periplasmic protein [synthetic construct],7DD9_G Chain G, Alpha-mannosidase,ZZ-type zinc finger-containing protein P35G2.11c,Maltose/maltodextrin-binding periplasmic protein [synthetic construct] |
| 5JM0_A | 4.57e-10 | 78 | 479 | 313 | 717 | Structureof the S. cerevisiae alpha-mannosidase 1 [Saccharomyces cerevisiae S288C] |
| 2WYH_A | 2.57e-08 | 54 | 898 | 28 | 921 | Structureof the Streptococcus pyogenes family GH38 alpha-mannosidase [Streptococcus pyogenes M1 GAS],2WYH_B Structure of the Streptococcus pyogenes family GH38 alpha-mannosidase [Streptococcus pyogenes M1 GAS],2WYI_A Structure of the Streptococcus pyogenes family GH38 alpha-mannosidase complexed with swainsonine [Streptococcus pyogenes M1 GAS],2WYI_B Structure of the Streptococcus pyogenes family GH38 alpha-mannosidase complexed with swainsonine [Streptococcus pyogenes M1 GAS] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q54K67 | 3.50e-14 | 61 | 896 | 264 | 1083 | Alpha-mannosidase G OS=Dictyostelium discoideum OX=44689 GN=manG PE=1 SV=1 |
| P54746 | 2.79e-13 | 55 | 883 | 19 | 854 | Mannosylglycerate hydrolase OS=Escherichia coli (strain K12) OX=83333 GN=mngB PE=1 SV=2 |
| Q9NTJ4 | 2.12e-10 | 36 | 593 | 229 | 732 | Alpha-mannosidase 2C1 OS=Homo sapiens OX=9606 GN=MAN2C1 PE=1 SV=1 |
| Q9UT61 | 8.37e-10 | 34 | 899 | 249 | 1077 | Alpha-mannosidase OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=ams1 PE=1 SV=1 |
| P22855 | 2.49e-09 | 78 | 479 | 300 | 704 | Alpha-mannosidase OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=AMS1 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
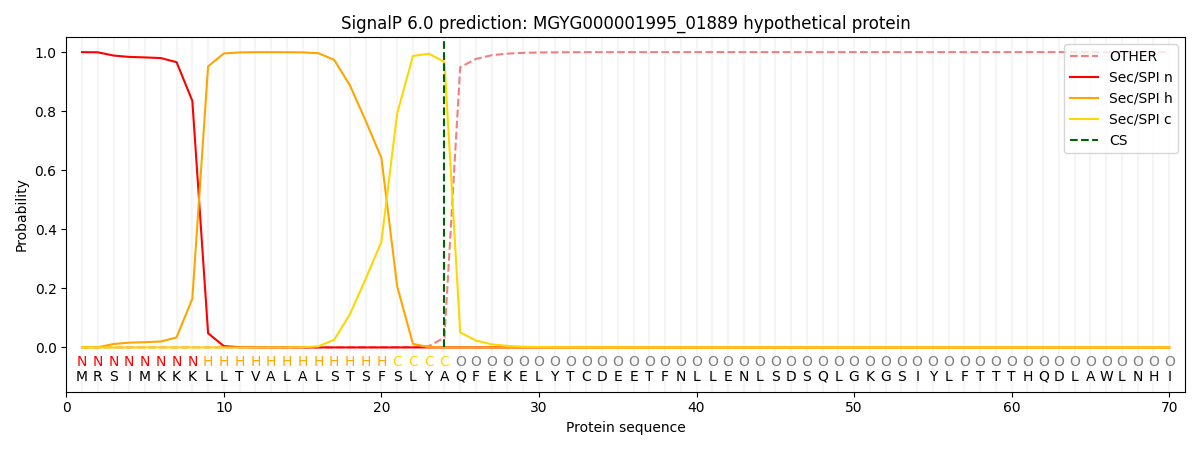
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000835 | 0.998128 | 0.000393 | 0.000223 | 0.000196 | 0.000174 |
