You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001998_00359
You are here: Home > Sequence: MGYG000001998_00359
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
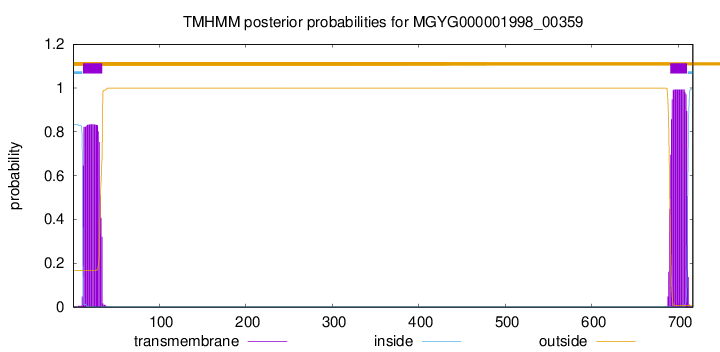
TMHMM annotations
Basic Information help
| Species | Lachnospira sp900552795 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Lachnospira; Lachnospira sp900552795 | |||||||||||
| CAZyme ID | MGYG000001998_00359 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 45353; End: 47506 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 177 | 430 | 6.8e-30 | 0.806930693069307 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00656 | Amb_all | 7.59e-15 | 166 | 391 | 6 | 167 | Amb_all domain. |
| COG3866 | PelB | 1.82e-09 | 181 | 478 | 104 | 321 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam00544 | Pec_lyase_C | 5.01e-05 | 170 | 268 | 28 | 134 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
| NF033847 | MCP_Sipho | 2.98e-04 | 585 | 683 | 8 | 107 | major capsid protein, Siphoviridae type. This protein is a phage major capsid protein, as reported in primary sequence submissions of a large number of Siphoviridae, many of which have hosts in the Mycobacterium and Gordonia genera of bacteria. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADL50775.1 | 2.52e-199 | 41 | 578 | 27 | 552 |
| BAV13078.1 | 2.78e-199 | 41 | 578 | 30 | 555 |
| QNF29855.1 | 3.53e-198 | 30 | 578 | 15 | 550 |
| QYR22341.1 | 1.89e-190 | 24 | 588 | 9 | 561 |
| AFH62972.1 | 7.04e-188 | 33 | 588 | 17 | 559 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5GT5_A | 4.11e-39 | 67 | 578 | 8 | 446 | Structuralbasis of the specific activity and thermostability of pectate lyase (pelN) from Paenibacillus sp. 0602 [Paenibacillus sp. 0602],5GT5_B Structural basis of the specific activity and thermostability of pectate lyase (pelN) from Paenibacillus sp. 0602 [Paenibacillus sp. 0602] |
| 3KRG_A | 7.86e-09 | 196 | 431 | 146 | 341 | ChainA, Pectate lyase [Bacillus subtilis] |
| 5AMV_A | 7.86e-09 | 196 | 431 | 146 | 341 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
| 1BN8_A | 8.41e-09 | 196 | 431 | 167 | 362 | BacillusSubtilis Pectate Lyase [Bacillus subtilis] |
| 2BSP_A | 1.95e-08 | 196 | 431 | 167 | 362 | ChainA, PROTEIN (PECTATE LYASE) [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| D3JTC2 | 2.26e-39 | 49 | 437 | 20 | 378 | Pectate lyase B OS=Paenibacillus amylolyticus OX=1451 GN=pelB PE=1 SV=1 |
| Q9WYR4 | 1.25e-09 | 177 | 284 | 79 | 210 | Pectate trisaccharide-lyase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=pelA PE=1 SV=1 |
| B1L969 | 3.86e-09 | 177 | 284 | 77 | 208 | Pectate trisaccharide-lyase OS=Thermotoga sp. (strain RQ2) OX=126740 GN=pelA PE=3 SV=1 |
| P39116 | 4.61e-08 | 196 | 431 | 167 | 362 | Pectate lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pel PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
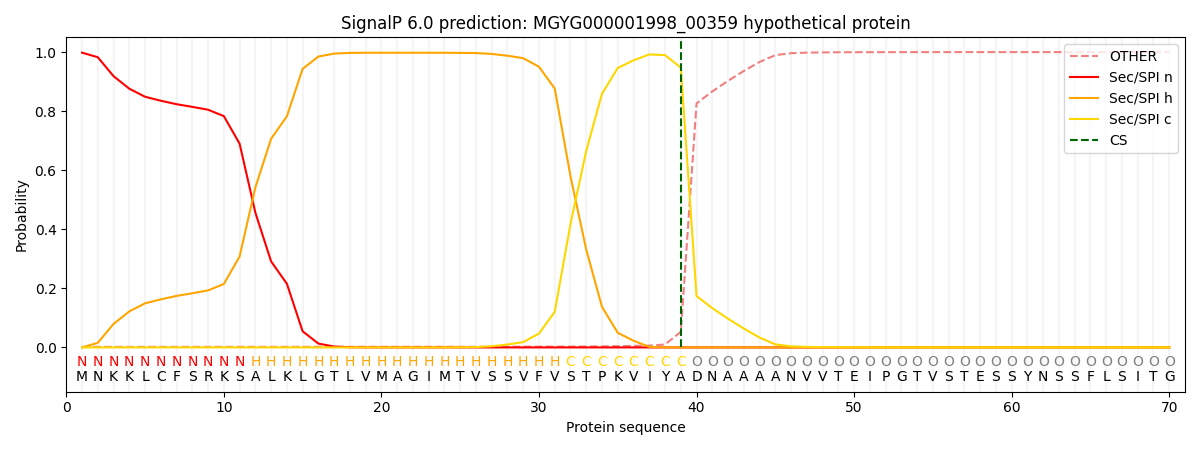
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002888 | 0.996033 | 0.000257 | 0.000360 | 0.000243 | 0.000200 |