You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002056_01358
You are here: Home > Sequence: MGYG000002056_01358
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-115 sp000432175 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; CAG-115; CAG-115 sp000432175 | |||||||||||
| CAZyme ID | MGYG000002056_01358 | |||||||||||
| CAZy Family | GH5 | |||||||||||
| CAZyme Description | Endoglucanase A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8973; End: 10118 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH5 | 76 | 346 | 1.1e-96 | 0.9927536231884058 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00150 | Cellulase | 7.49e-68 | 67 | 349 | 1 | 272 | Cellulase (glycosyl hydrolase family 5). |
| COG2730 | BglC | 1.10e-35 | 2 | 367 | 4 | 382 | Aryl-phospho-beta-D-glucosidase BglC, GH1 family [Carbohydrate transport and metabolism]. |
| cd03174 | DRE_TIM_metallolyase | 0.009 | 85 | 143 | 75 | 135 | DRE-TIM metallolyase superfamily. The DRE-TIM metallolyase superfamily includes 2-isopropylmalate synthase (IPMS), alpha-isopropylmalate synthase (LeuA), 3-hydroxy-3-methylglutaryl-CoA lyase, homocitrate synthase, citramalate synthase, 4-hydroxy-2-oxovalerate aldolase, re-citrate synthase, transcarboxylase 5S, pyruvate carboxylase, AksA, and FrbC. These members all share a conserved triose-phosphate isomerase (TIM) barrel domain consisting of a core beta(8)-alpha(8) motif with the eight parallel beta strands forming an enclosed barrel surrounded by eight alpha helices. The domain has a catalytic center containing a divalent cation-binding site formed by a cluster of invariant residues that cap the core of the barrel. In addition, the catalytic site includes three invariant residues - an aspartate (D), an arginine (R), and a glutamate (E) - which is the basis for the domain name "DRE-TIM". |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADK66823.1 | 2.08e-118 | 34 | 376 | 18 | 364 |
| CBL17440.1 | 4.28e-113 | 13 | 381 | 10 | 379 |
| CBL16772.1 | 3.12e-112 | 52 | 376 | 101 | 437 |
| CDM69884.1 | 4.05e-104 | 59 | 375 | 49 | 375 |
| AOZ95201.1 | 8.41e-101 | 30 | 379 | 61 | 417 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6WQP_A | 5.56e-114 | 52 | 376 | 18 | 354 | GH5-4broad specificity endoglucanase from Ruminococcus champanellensis [Ruminococcus champanellensis],6WQP_B GH5-4 broad specificity endoglucanase from Ruminococcus champanellensis [Ruminococcus champanellensis],6WQV_A GH5-4 broad specificity endoglucanase from Ruminococcus champanellensis with bound cellotriose [Ruminococcus champanellensis],6WQV_B GH5-4 broad specificity endoglucanase from Ruminococcus champanellensis with bound cellotriose [Ruminococcus champanellensis],6WQV_C GH5-4 broad specificity endoglucanase from Ruminococcus champanellensis with bound cellotriose [Ruminococcus champanellensis],6WQV_D GH5-4 broad specificity endoglucanase from Ruminococcus champanellensis with bound cellotriose [Ruminococcus champanellensis] |
| 6Q1I_A | 1.86e-102 | 52 | 379 | 16 | 354 | GH5-4broad specificity endoglucanase from Clostrdium longisporum [Clostridium longisporum],6Q1I_B GH5-4 broad specificity endoglucanase from Clostrdium longisporum [Clostridium longisporum] |
| 4IM4_A | 2.79e-99 | 48 | 378 | 5 | 335 | ChainA, Endoglucanase E [Acetivibrio thermocellus],4IM4_B Chain B, Endoglucanase E [Acetivibrio thermocellus],4IM4_C Chain C, Endoglucanase E [Acetivibrio thermocellus],4IM4_D Chain D, Endoglucanase E [Acetivibrio thermocellus],4IM4_E Chain E, Endoglucanase E [Acetivibrio thermocellus],4IM4_F Chain F, Endoglucanase E [Acetivibrio thermocellus] |
| 3NDY_A | 3.73e-99 | 52 | 376 | 14 | 339 | Thestructure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans [Clostridium cellulovorans],3NDY_B The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans [Clostridium cellulovorans],3NDY_C The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans [Clostridium cellulovorans],3NDY_D The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans [Clostridium cellulovorans],3NDZ_A The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans bound to cellotriose [Clostridium cellulovorans],3NDZ_B The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans bound to cellotriose [Clostridium cellulovorans],3NDZ_C The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans bound to cellotriose [Clostridium cellulovorans],3NDZ_D The structure of the catalytic and carbohydrate binding domain of endoglucanase D from Clostridium cellulovorans bound to cellotriose [Clostridium cellulovorans] |
| 6MQ4_A | 6.23e-97 | 48 | 378 | 10 | 349 | ChainA, cellulase [Acetivibrio cellulolyticus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q12647 | 2.28e-101 | 52 | 376 | 27 | 361 | Endoglucanase B OS=Neocallimastix patriciarum OX=4758 GN=CELB PE=2 SV=1 |
| P54937 | 1.63e-100 | 52 | 379 | 41 | 379 | Endoglucanase A OS=Clostridium longisporum OX=1523 GN=celA PE=1 SV=1 |
| P23660 | 8.23e-100 | 48 | 379 | 25 | 364 | Endoglucanase A OS=Ruminococcus albus OX=1264 GN=celA PE=1 SV=1 |
| P28623 | 1.70e-96 | 52 | 376 | 45 | 370 | Endoglucanase D OS=Clostridium cellulovorans (strain ATCC 35296 / DSM 3052 / OCM 3 / 743B) OX=573061 GN=engD PE=1 SV=2 |
| P23661 | 2.20e-94 | 4 | 379 | 3 | 401 | Endoglucanase B OS=Ruminococcus albus OX=1264 GN=celB PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
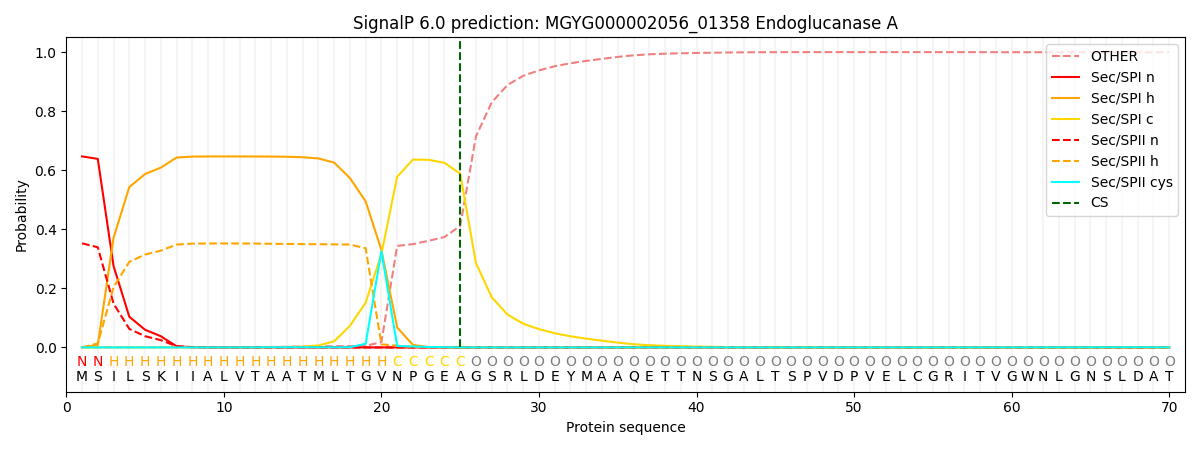
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001927 | 0.640366 | 0.357021 | 0.000271 | 0.000216 | 0.000189 |
