You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002179_00298
You are here: Home > Sequence: MGYG000002179_00298
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; UBA932; CAG-831; | |||||||||||
| CAZyme ID | MGYG000002179_00298 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 81266; End: 83659 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 361 | 697 | 1e-90 | 0.8959537572254336 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00812 | Alpha_L_fucos | 6.36e-123 | 349 | 736 | 2 | 384 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
| pfam01120 | Alpha_L_fucos | 1.97e-91 | 363 | 692 | 15 | 332 | Alpha-L-fucosidase. |
| COG3669 | AfuC | 1.22e-33 | 380 | 697 | 1 | 321 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
| cd09078 | nSMase | 7.20e-12 | 29 | 341 | 3 | 280 | Neutral sphingomyelinases (nSMase) catalyze the hydrolysis of sphingomyelin in biological membranes to ceramide and phosphorylcholine. Sphingomyelinases (SMase) are phosphodiesterases that catalyze the hydrolysis of sphingomyelin to ceramide and phosphorylcholine. Eukaryotic SMases have been classified according to their pH optima and are known as acid SMase, alkaline SMase, and neutral SMase (nSMase). Eukaryotic proteins in this family are nSMases, and are activated by a variety of stress-inducing agents such as cytokines or UV radiation. Ceramides and other metabolic derivatives, including sphingosine, are lipid "second messenger" molecules that participate in the regulation of stress-induced cellular responses, including cell death, adhesion, differentiation, and proliferation. Bacterial neutral SMases, which also belong to this domain family, are secreted proteins that act as membrane-damaging virulence factors. They promote colonization of the host tissue. This family belongs to the large EEP (exonuclease/endonuclease/phosphatase) superfamily that contains functionally diverse enzymes that share a common catalytic mechanism of cleaving phosphodiester bonds. |
| cd09080 | TDP2 | 2.10e-09 | 27 | 341 | 1 | 248 | Phosphodiesterase domain of human TDP2, a 5'-tyrosyl DNA phosphodiesterase, and related domains. Human TDP2, also known as TTRAP (TRAF/TNFR-associated factors, and tumor necrosis factor receptor/TNFR-associated protein), is a 5'-tyrosyl DNA phosphodiesterase. It is required for the efficient repair of topoisomerase II-induced DNA double strand breaks. The topoisomerase is covalently linked by a phosphotyrosyl bond to the 5'-terminus of the break. TDP2 cleaves the DNA 5'-phosphodiester bond and restores 5'-phosphate termini, needed for subsequent DNA ligation, and hence repair of the break. TDP2 and 3'-tyrosyl DNA phosphodiesterase (TDP1) are complementary activities; together, they allow cells to remove trapped topoisomerase from both 3'- and 5'-DNA termini. TTRAP has been reported as being involved in apoptosis, embryonic development, and transcriptional regulation, and it may inhibit the activation of nuclear factor-kB. This family belongs to the large EEP (exonuclease/endonuclease/phosphatase) superfamily that contains functionally diverse enzymes that share a common catalytic mechanism of cleaving phosphodiester bonds. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANQ62400.1 | 1.12e-281 | 348 | 796 | 24 | 471 |
| CAH05807.1 | 1.12e-281 | 348 | 796 | 24 | 471 |
| AKA53992.1 | 1.12e-281 | 348 | 796 | 24 | 471 |
| QCT76666.1 | 1.12e-281 | 348 | 796 | 24 | 471 |
| QUU05154.1 | 1.12e-281 | 348 | 796 | 24 | 471 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2XIB_A | 2.23e-275 | 346 | 796 | 1 | 452 | CrystalStructure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With Deoxyfuconojirimycin [Bacteroides thetaiotaomicron],2XIB_B Crystal Structure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With Deoxyfuconojirimycin [Bacteroides thetaiotaomicron],2XIB_C Crystal Structure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With Deoxyfuconojirimycin [Bacteroides thetaiotaomicron],2XIB_D Crystal Structure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With Deoxyfuconojirimycin [Bacteroides thetaiotaomicron],2XII_A Crystal Structure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With An Extended 9- Fluorenone Iminosugar Inhibitor [Bacteroides thetaiotaomicron],2XII_B Crystal Structure Of An Alpha-L-Fucosidase Gh29 From Bacteroides Thetaiotaomicron In Complex With An Extended 9- Fluorenone Iminosugar Inhibitor [Bacteroides thetaiotaomicron] |
| 4JL2_A | 4.01e-275 | 349 | 796 | 1 | 449 | Crystalstructure of a bacterial fucosidase with a monovalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JL2_B Crystal structure of a bacterial fucosidase with a monovalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482] |
| 6HZY_A | 5.07e-275 | 346 | 796 | 23 | 474 | Crystalstructure of a bacterial fucosidase with inhibitor FucPUG [Bacteroides thetaiotaomicron VPI-5482],6HZY_B Crystal structure of a bacterial fucosidase with inhibitor FucPUG [Bacteroides thetaiotaomicron VPI-5482] |
| 2WVV_B | 1.14e-274 | 349 | 796 | 1 | 449 | Crystalstructure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482] |
| 2WVV_A | 1.62e-274 | 349 | 796 | 1 | 449 | Crystalstructure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],2WVV_C Crystal structure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],2WVV_D Crystal structure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],4J27_A Crystal structure of a gh29 alpha-l-fucosidase gh29 from bacteroides thetaiotaomicron in a novel crystal form [Bacteroides thetaiotaomicron VPI-5482],4J27_B Crystal structure of a gh29 alpha-l-fucosidase gh29 from bacteroides thetaiotaomicron in a novel crystal form [Bacteroides thetaiotaomicron VPI-5482],4J28_A Crystal structure of a gh29 alpha-l-fucosidase gh29 from bacteroides thetaiotaomicron in complex with a 5-membered iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482],4J28_B Crystal structure of a gh29 alpha-l-fucosidase gh29 from bacteroides thetaiotaomicron in complex with a 5-membered iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JFS_A Crystal structure of a bacterial fucosidase with iminosugar inhibitor 4-epi-(+)-Codonopsinine [Bacteroides thetaiotaomicron VPI-5482],4JFS_B Crystal structure of a bacterial fucosidase with iminosugar inhibitor 4-epi-(+)-Codonopsinine [Bacteroides thetaiotaomicron VPI-5482],4JFT_A Crystal structure of a bacterial fucosidase with iminosugar inhibitor N-desmethyl-4-epi-(+)-Codonopsinine [Bacteroides thetaiotaomicron VPI-5482],4JFT_B Crystal structure of a bacterial fucosidase with iminosugar inhibitor N-desmethyl-4-epi-(+)-Codonopsinine [Bacteroides thetaiotaomicron VPI-5482],4JFU_A Crystal structure of a bacterial fucosidase with iminosugar inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JFU_B Crystal structure of a bacterial fucosidase with iminosugar inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JFV_A Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(methylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFV_B Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(methylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFV_C Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(methylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFV_D Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(methylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFW_A Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(propylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFW_B Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(propylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFW_C Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(propylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JFW_D Crystal structure of a bacterial fucosidase with iminosugar inhibitor (2S,3S,4R,5S)-2-[N-(propylferrocene)]aminoethyl-5-methylpyrrolidine-3,4-diol [Bacteroides thetaiotaomicron VPI-5482],4JL1_A Crystal structure of a bacterial fucosidase with a multivalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JL1_B Crystal structure of a bacterial fucosidase with a multivalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P17164 | 1.64e-41 | 330 | 773 | 9 | 446 | Tissue alpha-L-fucosidase OS=Rattus norvegicus OX=10116 GN=Fuca1 PE=1 SV=1 |
| P04066 | 1.77e-41 | 351 | 747 | 34 | 424 | Tissue alpha-L-fucosidase OS=Homo sapiens OX=9606 GN=FUCA1 PE=1 SV=4 |
| Q60HF8 | 4.60e-41 | 342 | 747 | 26 | 426 | Tissue alpha-L-fucosidase OS=Macaca fascicularis OX=9541 GN=FUCA1 PE=2 SV=1 |
| C3YWU0 | 4.31e-39 | 363 | 781 | 27 | 446 | Alpha-L-fucosidase OS=Branchiostoma floridae OX=7739 GN=BRAFLDRAFT_56888 PE=3 SV=2 |
| Q99LJ1 | 2.08e-38 | 351 | 747 | 20 | 410 | Tissue alpha-L-fucosidase OS=Mus musculus OX=10090 GN=Fuca1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
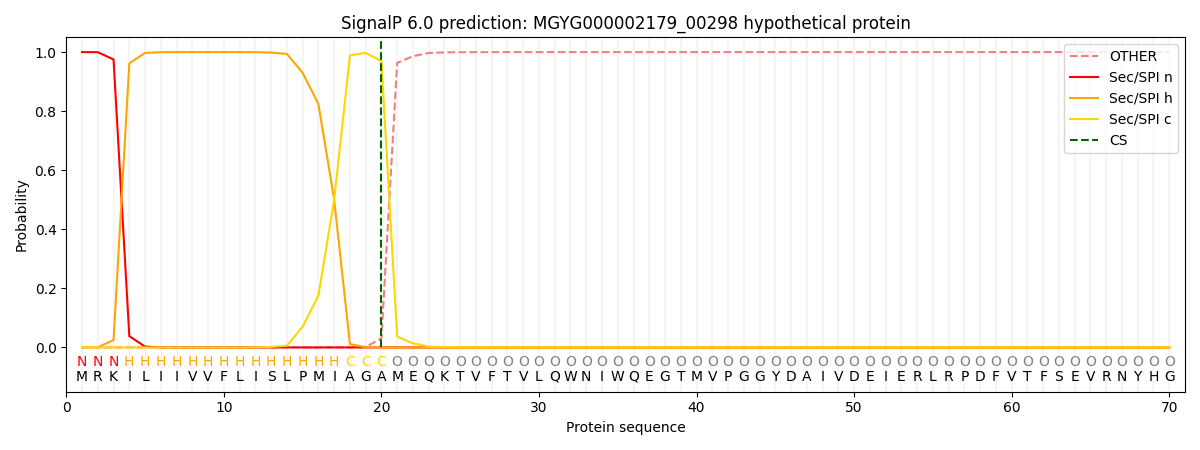
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000249 | 0.999134 | 0.000172 | 0.000151 | 0.000140 | 0.000137 |
