You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002213_01276
You are here: Home > Sequence: MGYG000002213_01276
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Oscillospiraceae; UMGS1872; | |||||||||||
| CAZyme ID | MGYG000002213_01276 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 5058; End: 5882 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 71 | 198 | 3e-34 | 0.9461538461538461 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| TIGR02884 | spore_pdaA | 6.10e-117 | 43 | 266 | 1 | 224 | delta-lactam-biosynthetic de-N-acetylase. Muramic delta-lactam is an unusual constituent of peptidoglycan, found only in bacterial spores in the peptidoglycan wall, or spore cortex. The proteins in this family are PdaA (yfjS), a member of a larger family of polysaccharide deacetylases, and are specificially involved in delta-lactam biosynthesis. PdaA acts immediately after CwlD, an N-acetylmuramoyl-L-alanine amidase and performs a de-N-acetylation. PdaA may also perform the following transpeptidation for lactam ring formation, as heterologous expression in E. coli of CwlD and PdaA together is sufficient for delta-lactam production. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan, Cellular processes, Sporulation and germination] |
| cd10948 | CE4_BsPdaA_like | 1.64e-110 | 43 | 262 | 4 | 223 | Catalytic NodB homology domain of Bacillus subtilis polysaccharide deacetylase PdaA, and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis pdaA gene encoding polysaccharide deacetylase BsPdaA, which is a member of the carbohydrate esterase 4 (CE4) superfamily. BsPdaA deacetylates peptidoglycan N-acetylmuramic acid (MurNAc) residues to facilitate the formation of muramic delta-lactam, which is required for recognition of germination lytic enzymes. BsPdaA deficiency leads to the absence of muramic delta-lactam residues in the spore cortex. Like other CE4 esterases, BsPdaA consists of a single catalytic NodB homology domain that appears to adopt a deformed (beta/alpha)8 barrel fold with a putative substrate binding groove harboring the majority of the conserved residues. It utilizes a general acid/base catalytic mechanism involving a tetrahedral transition intermediate, where a water molecule functions as the nucleophile tightly associated to the zinc cofactor. |
| cd10917 | CE4_NodB_like_6s_7s | 8.25e-61 | 78 | 250 | 1 | 168 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| cd10950 | CE4_BsYlxY_like | 7.57e-48 | 73 | 265 | 1 | 187 | Putative catalytic NodB homology domain of uncharacterized protein YlxY from Bacillus subtilis and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis putative polysaccharide deacetylase BsYlxY, encoded by the ylxY gene, which is a member of the carbohydrate esterase 4 (CE4) superfamily. Although its biological function still remains unknown, BsYlxY shows high sequence homology to the catalytic domain of Bacillus subtilis pdaB gene encoding a putative polysaccharide deacetylase (BsPdaB), which is essential for the maintenance of spores after the late stage of sporulation and is highly conserved in spore-forming bacteria. However, disruption of the ylxY gene in B. subtilis did not cause any sporulation defect. Moreover, the Asp residue in the classical His-His-Asp zinc-binding motif of CE4 esterases is mutated to a Val residue in this family. Other catalytically relevant residues of CE4 esterases are also not conserved, which suggest that members of this family may be inactive. |
| cd10962 | CE4_GT2-like | 9.42e-48 | 78 | 265 | 1 | 194 | Catalytic NodB homology domain of uncharacterized bacterial glycosyl transferase, group 2-like family proteins. This family includes many uncharacterized bacterial proteins containing an N-terminal GH18 (glycosyl hydrolase, family 18) domain, a middle NodB-like homology domain, and a C-terminal GT2-like (glycosyl transferase group 2) domain. Although their biological function is unknown, members in this family contain a middle NodB homology domain that is similar to the catalytic domain of Streptococcus pneumoniae polysaccharide deacetylase PgdA (SpPgdA), an extracellular metal-dependent polysaccharide deacetylase with de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. Like SpPgdA, this family is a member of the carbohydrate esterase 4 (CE4) superfamily. The presence of three domains suggests that members of this family may be multifunctional. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUO39071.1 | 6.32e-141 | 29 | 266 | 34 | 271 |
| BCK85659.1 | 2.17e-139 | 31 | 266 | 33 | 268 |
| QCI60928.1 | 3.44e-135 | 36 | 266 | 32 | 261 |
| QIB56171.1 | 1.99e-117 | 4 | 274 | 7 | 277 |
| QMW81094.1 | 1.99e-117 | 4 | 274 | 7 | 277 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1W1A_1 | 7.55e-68 | 36 | 265 | 17 | 246 | Structureof Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1A_2 Structure of Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_1 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_2 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1W17_A | 9.08e-68 | 36 | 265 | 23 | 252 | Structureof Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W17_B Structure of Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1NY1_A | 2.88e-66 | 37 | 265 | 1 | 229 | CrystalStructure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis],1NY1_B Crystal Structure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis] |
| 2J13_A | 8.41e-62 | 43 | 265 | 19 | 241 | Structureof a family 4 carbohydrate esterase from Bacillus anthracis [Bacillus anthracis str. Ames] |
| 5O6Y_A | 1.08e-30 | 67 | 265 | 10 | 207 | Crystalstructure of the Bc1960 peptidoglycan N-acetylglucosamine deacetylase in complex with 4-naphthalen-1-yl-~{N}-oxidanyl-benzamide [Bacillus cereus ATCC 14579] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q04729 | 2.32e-68 | 35 | 265 | 23 | 253 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
| O34928 | 4.97e-67 | 36 | 265 | 23 | 252 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaA PE=1 SV=1 |
| Q81EK9 | 2.45e-29 | 67 | 265 | 70 | 267 | Peptidoglycan-N-acetylglucosamine deacetylase BC_1960 OS=Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG 7414 / JCM 2152 / NBRC 15305 / NCIMB 9373 / NCTC 2599 / NRRL B-3711) OX=226900 GN=BC_1960 PE=1 SV=1 |
| P50850 | 6.02e-28 | 71 | 269 | 123 | 315 | Uncharacterized protein YlxY OS=Bacillus subtilis (strain 168) OX=224308 GN=ylxY PE=3 SV=2 |
| A0A0H3GDH9 | 1.16e-27 | 77 | 265 | 265 | 445 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain 10403S) OX=393133 GN=pgdA PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
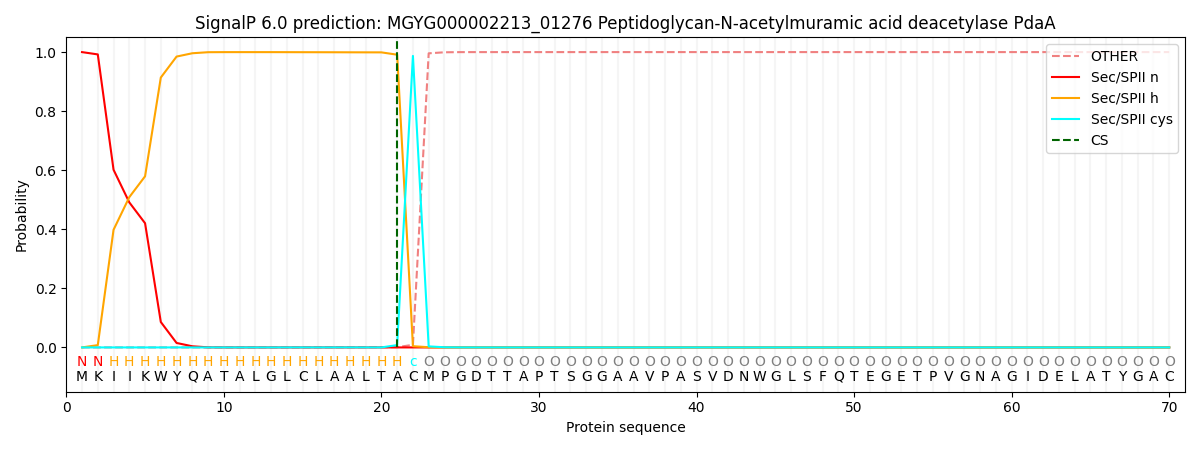
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000038 | 0.999995 | 0.000000 | 0.000000 | 0.000000 |
