You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002281_02098
You are here: Home > Sequence: MGYG000002281_02098
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides faecis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides faecis | |||||||||||
| CAZyme ID | MGYG000002281_02098 | |||||||||||
| CAZy Family | GH30 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 37782; End: 39239 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH30 | 47 | 483 | 6.1e-179 | 0.9928057553956835 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam02055 | Glyco_hydro_30 | 5.24e-34 | 58 | 418 | 2 | 348 | Glycosyl hydrolase family 30 TIM-barrel domain. |
| COG5520 | XynC | 4.81e-29 | 41 | 483 | 31 | 427 | O-Glycosyl hydrolase [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QIU97167.1 | 0.0 | 2 | 485 | 12 | 495 |
| QUT89026.1 | 1.10e-285 | 6 | 485 | 14 | 492 |
| ALJ59961.1 | 6.05e-283 | 6 | 485 | 14 | 492 |
| QDO68217.1 | 3.29e-278 | 14 | 485 | 23 | 493 |
| AVM56494.1 | 4.38e-261 | 1 | 485 | 16 | 498 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2WNW_A | 1.94e-22 | 60 | 483 | 48 | 441 | Thecrystal structure of SrfJ from salmonella typhimurium [Salmonella enterica subsp. enterica serovar Typhimurium str. LT2],2WNW_B The crystal structure of SrfJ from salmonella typhimurium [Salmonella enterica subsp. enterica serovar Typhimurium str. LT2] |
| 5NGK_A | 6.69e-21 | 34 | 482 | 55 | 488 | Theendo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGK_B The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGK_C The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_A The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_B The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_C The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron] |
| 1OGS_A | 2.25e-17 | 71 | 464 | 90 | 473 | humanacid-beta-glucosidase [Homo sapiens],1OGS_B human acid-beta-glucosidase [Homo sapiens],1Y7V_A Chain A, Glucosylceramidase [Homo sapiens],1Y7V_B Chain B, Glucosylceramidase [Homo sapiens],2F61_A Crystal structure of partially deglycosylated acid beta-glucosidase [Homo sapiens],2F61_B Crystal structure of partially deglycosylated acid beta-glucosidase [Homo sapiens],2J25_A Partially deglycosylated glucoceramidase [Homo sapiens],2J25_B Partially deglycosylated glucoceramidase [Homo sapiens],2NSX_A Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_B Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_C Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_D Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NT0_A Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_B Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_C Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_D Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT1_A Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_B Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_C Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_D Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],3GXD_A Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_B Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_C Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_D Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXF_A Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_B Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_C Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_D Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXI_A Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_B Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_C Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_D Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXM_A Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_B Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_C Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_D Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3RIK_A The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_B The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_C The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_D The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_A The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_B The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_C The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_D The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],6MOZ_A Structure of acid-beta-glucosidase in complex with an aromatic pyrrolidine iminosugar inhibitor [Homo sapiens],6MOZ_B Structure of acid-beta-glucosidase in complex with an aromatic pyrrolidine iminosugar inhibitor [Homo sapiens],6Q1N_A Glucocerebrosidase in complex with pharmacological chaperone IMX8 [Homo sapiens],6Q1N_B Glucocerebrosidase in complex with pharmacological chaperone IMX8 [Homo sapiens],6Q1P_A Glucocerebrosidase in complex with pharmacological chaperone norIMX8 [Homo sapiens],6Q1P_B Glucocerebrosidase in complex with pharmacological chaperone norIMX8 [Homo sapiens],6Q6K_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with cyclophellitol activity based probe with Cy5 tag (ME569) [Homo sapiens],6Q6K_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with cyclophellitol activity based probe with Cy5 tag (ME569) [Homo sapiens],6Q6L_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with adamantyl-cyclophellitol inhibitor (ME656) [Homo sapiens],6Q6L_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with adamantyl-cyclophellitol inhibitor (ME656) [Homo sapiens],6Q6N_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with biphenyl-cyclophellitol inhibitor (ME655) [Homo sapiens],6Q6N_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with biphenyl-cyclophellitol inhibitor (ME655) [Homo sapiens],6TJJ_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6TJJ_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YTP_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YTP_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YUT_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YUT_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YV3_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YV3_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6Z39_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6Z39_BBB Chain BBB, Glucosylceramidase [Homo sapiens] |
| 2WKL_A | 2.25e-17 | 71 | 464 | 90 | 473 | Velaglucerasealfa [Homo sapiens],2WKL_B Velaglucerase alfa [Homo sapiens],5LVX_A Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_B Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_C Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_D Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],6TJK_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6TJK_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],6TJQ_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6TN1_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6YTR_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6YTR_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],6Z3I_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],7NWV_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],7NWV_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens] |
| 2V3D_A | 2.31e-17 | 71 | 464 | 92 | 475 | acid-beta-glucosidasewith N-butyl-deoxynojirimycin [Homo sapiens],2V3D_B acid-beta-glucosidase with N-butyl-deoxynojirimycin [Homo sapiens],2V3E_A acid-beta-glucosidase with N-nonyl-deoxynojirimycin [Homo sapiens],2V3E_B acid-beta-glucosidase with N-nonyl-deoxynojirimycin [Homo sapiens],2V3F_A acid-beta-glucosidase produced in carrot [Homo sapiens],2V3F_B acid-beta-glucosidase produced in carrot [Homo sapiens],2VT0_A X-ray structure of a conjugate with conduritol-beta-epoxide of acid-beta-glucosidase overexpressed in cultured plant cells [Homo sapiens],2VT0_B X-ray structure of a conjugate with conduritol-beta-epoxide of acid-beta-glucosidase overexpressed in cultured plant cells [Homo sapiens],2WCG_A X-ray structure of acid-beta-glucosidase with N-octyl(cyclic guanidine)-nojirimycin in the active site [Homo sapiens],2WCG_B X-ray structure of acid-beta-glucosidase with N-octyl(cyclic guanidine)-nojirimycin in the active site [Homo sapiens],2XWD_A X-Ray Structure Of Acid-Beta-Glucosidase With 5n,6o-(N'-(N- Octyl)imino)nojirimycin In The Active Site [Homo sapiens],2XWD_B X-Ray Structure Of Acid-Beta-Glucosidase With 5n,6o-(N'-(N- Octyl)imino)nojirimycin In The Active Site [Homo sapiens],2XWE_A X-ray Structure Of Acid-beta-glucosidase With 5n,6s-(n'-(n- Octyl)imino)-6-thionojirimycin In The Active Site [Homo sapiens],2XWE_B X-ray Structure Of Acid-beta-glucosidase With 5n,6s-(n'-(n- Octyl)imino)-6-thionojirimycin In The Active Site [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O16580 | 2.94e-26 | 46 | 422 | 87 | 449 | Putative glucosylceramidase 1 OS=Caenorhabditis elegans OX=6239 GN=gba-1 PE=1 SV=2 |
| G5ECR8 | 3.94e-26 | 22 | 485 | 65 | 519 | Putative glucosylceramidase 3 OS=Caenorhabditis elegans OX=6239 GN=gba-3 PE=3 SV=1 |
| Q9UB00 | 1.39e-24 | 20 | 422 | 62 | 451 | Putative glucosylceramidase 4 OS=Caenorhabditis elegans OX=6239 GN=gba-4 PE=3 SV=2 |
| O16581 | 1.84e-24 | 48 | 455 | 89 | 481 | Putative glucosylceramidase 2 OS=Caenorhabditis elegans OX=6239 GN=gba-2 PE=3 SV=2 |
| P17439 | 3.08e-19 | 71 | 483 | 109 | 511 | Lysosomal acid glucosylceramidase OS=Mus musculus OX=10090 GN=Gba PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
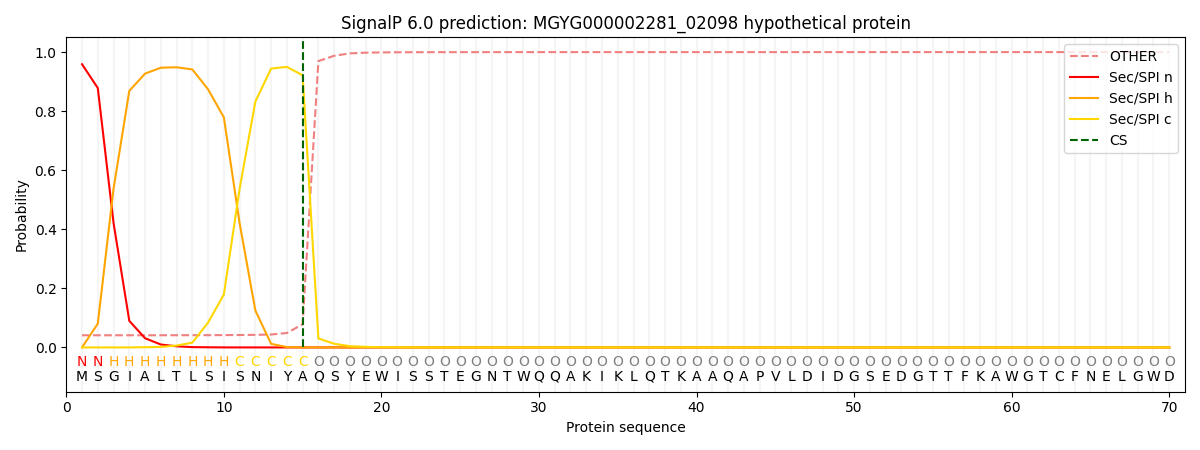
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.044597 | 0.954236 | 0.000255 | 0.000354 | 0.000266 | 0.000252 |
