You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002283_02412
You are here: Home > Sequence: MGYG000002283_02412
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacillus_A paranthracis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Bacillales; Bacillaceae_G; Bacillus_A; Bacillus_A paranthracis | |||||||||||
| CAZyme ID | MGYG000002283_02412 | |||||||||||
| CAZy Family | CBM50 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2327274; End: 2329001 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG5632 | CwlA | 3.06e-37 | 230 | 391 | 1 | 165 | N-acetylmuramoyl-L-alanine amidase CwlA [Cell wall/membrane/envelope biogenesis]. |
| cd06583 | PGRP | 3.91e-27 | 252 | 378 | 1 | 126 | Peptidoglycan recognition proteins (PGRPs) are pattern recognition receptors that bind, and in certain cases, hydrolyze peptidoglycans (PGNs) of bacterial cell walls. PGRPs have been divided into three classes: short PGRPs (PGRP-S), that are small (20 kDa) extracellular proteins; intermediate PGRPs (PGRP-I) that are 40-45 kDa and are predicted to be transmembrane proteins; and long PGRPs (PGRP-L), up to 90 kDa, which may be either intracellular or transmembrane. Several structures of PGRPs are known in insects and mammals, some bound with substrates like Muramyl Tripeptide (MTP) or Tracheal Cytotoxin (TCT). The substrate binding site is conserved in PGRP-LCx, PGRP-LE, and PGRP-Ialpha proteins. This family includes Zn-dependent N-Acetylmuramoyl-L-alanine Amidase, EC:3.5.1.28. This enzyme cleaves the amide bond between N-acetylmuramoyl and L-amino acids, preferentially D-lactyl-L-Ala, in bacterial cell walls. The structure for the bacteriophage T7 lysozyme shows that two of the conserved histidines and a cysteine are zinc binding residues. Site-directed mutagenesis of T7 lysozyme indicates that two conserved residues, a Tyr and a Lys, are important for amidase activity. |
| pfam01510 | Amidase_2 | 1.51e-24 | 252 | 375 | 1 | 119 | N-acetylmuramoyl-L-alanine amidase. This family includes zinc amidases that have N-acetylmuramoyl-L-alanine amidase activity EC:3.5.1.28. This enzyme domain cleaves the amide bond between N-acetylmuramoyl and L-amino acids in bacterial cell walls (preferentially: D-lactyl-L-Ala). The structure is known for the bacteriophage T7 structure and shows that two of the conserved histidines are zinc binding. |
| smart00644 | Ami_2 | 2.70e-22 | 251 | 375 | 1 | 126 | Ami_2 domain. |
| NF033190 | inl_like_NEAT_1 | 2.25e-12 | 400 | 570 | 583 | 753 | NEAT domain-containing leucine-rich repeat protein. Members of this family have an N-terminal NEAT (near transporter) domain often associated with iron transport, followed by a leucine-rich repeat region with significant sequence similarity to the internalins of Listeria monocytogenes. However, since Bacillus cereus (from which this protein was described, in PMID:16978259) is not considered an intracellular pathogen, and the function may be iron transport rather than internalization, applying the name "internalin" to this family probably would be misleading. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QTR69063.1 | 7.97e-248 | 1 | 575 | 1 | 591 |
| QTR76806.1 | 7.97e-248 | 1 | 575 | 1 | 591 |
| QTR85044.1 | 1.60e-247 | 1 | 575 | 1 | 591 |
| QTR73010.1 | 1.60e-247 | 1 | 575 | 1 | 591 |
| QTR89055.1 | 1.60e-247 | 1 | 575 | 1 | 591 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3HMB_A | 4.00e-49 | 231 | 390 | 5 | 155 | ChainA, N-acetylmuramoyl-L-alanine amidase xlyA [Bacillus subtilis],3HMB_B Chain B, N-acetylmuramoyl-L-alanine amidase xlyA [Bacillus subtilis],3HMB_C Chain C, N-acetylmuramoyl-L-alanine amidase xlyA [Bacillus subtilis] |
| 3RDR_A | 5.82e-47 | 238 | 390 | 12 | 155 | Structureof the catalytic domain of XlyA [Bacillus subtilis] |
| 1YB0_A | 7.86e-31 | 234 | 392 | 5 | 155 | Structureof PlyL [Bacillus anthracis],1YB0_B Structure of PlyL [Bacillus anthracis],1YB0_C Structure of PlyL [Bacillus anthracis] |
| 2L47_A | 9.33e-31 | 233 | 392 | 3 | 155 | Solutionstructure of the PlyG catalytic domain [Bacillus phage Gamma] |
| 2AR3_A | 5.44e-30 | 234 | 392 | 5 | 155 | ChainA, prophage lambdaba02, n-acetylmuramoyl-l-alanine amidase, family 2 [Bacillus anthracis],2AR3_B Chain B, prophage lambdaba02, n-acetylmuramoyl-l-alanine amidase, family 2 [Bacillus anthracis],2AR3_C Chain C, prophage lambdaba02, n-acetylmuramoyl-l-alanine amidase, family 2 [Bacillus anthracis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P54450 | 1.91e-46 | 231 | 390 | 2 | 153 | N-acetylmuramoyl-L-alanine amidase CwlH OS=Bacillus subtilis (strain 168) OX=224308 GN=cwlH PE=1 SV=1 |
| P39800 | 1.87e-44 | 238 | 390 | 9 | 152 | N-acetylmuramoyl-L-alanine amidase XlyA OS=Bacillus subtilis (strain 168) OX=224308 GN=xlyA PE=1 SV=1 |
| P14892 | 4.39e-28 | 233 | 379 | 3 | 143 | N-acetylmuramoyl-L-alanine amidase CwlA OS=Bacillus sp. OX=1409 GN=cwlA PE=3 SV=1 |
| O34391 | 6.20e-27 | 231 | 385 | 3 | 151 | N-acetylmuramoyl-L-alanine amidase XlyB OS=Bacillus subtilis (strain 168) OX=224308 GN=xlyB PE=3 SV=1 |
| P24808 | 2.18e-26 | 237 | 384 | 9 | 150 | N-acetylmuramoyl-L-alanine amidase CwlA OS=Bacillus subtilis (strain 168) OX=224308 GN=cwlA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
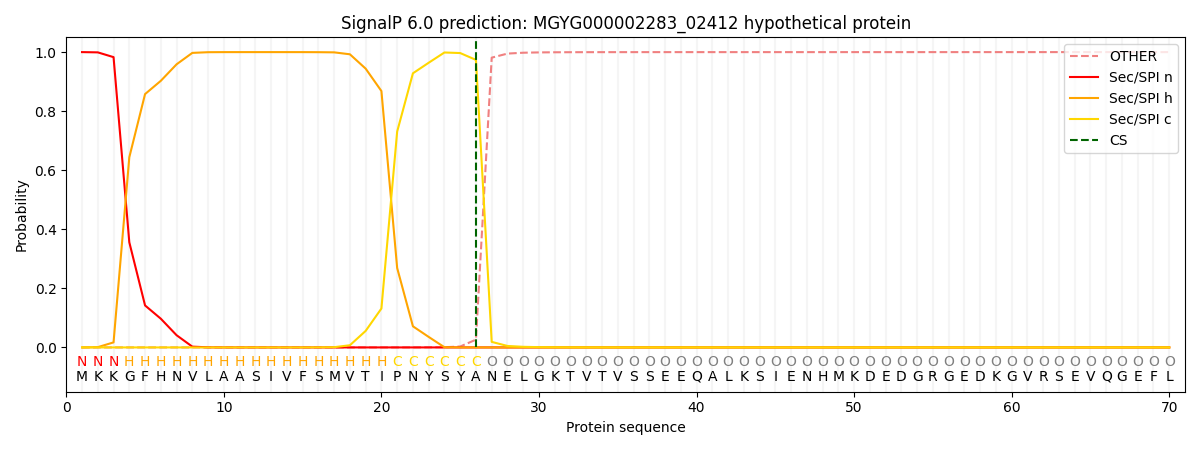
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000304 | 0.998932 | 0.000202 | 0.000183 | 0.000177 | 0.000159 |
