You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002353_00514
You are here: Home > Sequence: MGYG000002353_00514
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
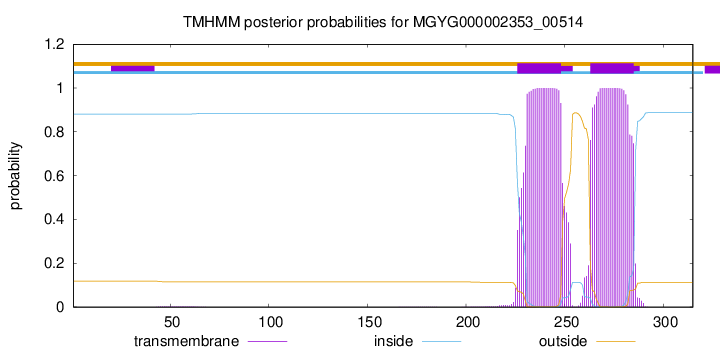
TMHMM annotations
Basic Information help
| Species | Enterococcus_B faecium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Lactobacillales; Enterococcaceae; Enterococcus_B; Enterococcus_B faecium | |||||||||||
| CAZyme ID | MGYG000002353_00514 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | Prophage bactoprenol glucosyl transferase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 537410; End: 538357 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 4 | 163 | 2.3e-31 | 0.9705882352941176 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd04187 | DPM1_like_bac | 1.80e-79 | 5 | 185 | 1 | 181 | Bacterial DPM1_like enzymes are related to eukaryotic DPM1. A family of bacterial enzymes related to eukaryotic DPM1; Although the mechanism of eukaryotic enzyme is well studied, the mechanism of the bacterial enzymes is not well understood. The eukaryotic DPM1 is the catalytic subunit of eukaryotic Dolichol-phosphate mannose (DPM) synthase. DPM synthase is required for synthesis of the glycosylphosphatidylinositol (GPI) anchor, N-glycan precursor, protein O-mannose, and C-mannose. The enzyme has three subunits, DPM1, DPM2 and DPM3. DPM is synthesized from dolichol phosphate and GDP-Man on the cytosolic surface of the ER membrane by DPM synthase and then is flipped onto the luminal side and used as a donor substrate. This protein family belongs to Glycosyltransferase 2 superfamily. |
| cd04179 | DPM_DPG-synthase_like | 3.95e-61 | 5 | 185 | 1 | 185 | DPM_DPG-synthase_like is a member of the Glycosyltransferase 2 superfamily. DPM1 is the catalytic subunit of eukaryotic dolichol-phosphate mannose (DPM) synthase. DPM synthase is required for synthesis of the glycosylphosphatidylinositol (GPI) anchor, N-glycan precursor, protein O-mannose, and C-mannose. In higher eukaryotes,the enzyme has three subunits, DPM1, DPM2 and DPM3. DPM is synthesized from dolichol phosphate and GDP-Man on the cytosolic surface of the ER membrane by DPM synthase and then is flipped onto the luminal side and used as a donor substrate. In lower eukaryotes, such as Saccharomyces cerevisiae and Trypanosoma brucei, DPM synthase consists of a single component (Dpm1p and TbDpm1, respectively) that possesses one predicted transmembrane region near the C terminus for anchoring to the ER membrane. In contrast, the Dpm1 homologues of higher eukaryotes, namely fission yeast, fungi, and animals, have no transmembrane region, suggesting the existence of adapter molecules for membrane anchoring. This family also includes bacteria and archaea DPM1_like enzymes. However, the enzyme structure and mechanism of function are not well understood. The UDP-glucose:dolichyl-phosphate glucosyltransferase (DPG_synthase) is a transmembrane-bound enzyme of the endoplasmic reticulum involved in protein N-linked glycosylation. This enzyme catalyzes the transfer of glucose from UDP-glucose to dolichyl phosphate. This protein family belongs to Glycosyltransferase 2 superfamily. |
| PRK10714 | PRK10714 | 3.55e-42 | 2 | 315 | 7 | 323 | undecaprenyl phosphate 4-deoxy-4-formamido-L-arabinose transferase; Provisional |
| pfam00535 | Glycos_transf_2 | 1.66e-30 | 4 | 163 | 1 | 164 | Glycosyl transferase family 2. Diverse family, transferring sugar from UDP-glucose, UDP-N-acetyl- galactosamine, GDP-mannose or CDP-abequose, to a range of substrates including cellulose, dolichol phosphate and teichoic acids. |
| COG0463 | WcaA | 2.20e-30 | 1 | 293 | 3 | 290 | Glycosyltransferase involved in cell wall bisynthesis [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QMX54164.1 | 3.27e-227 | 1 | 315 | 1 | 315 |
| QCV42866.1 | 3.27e-227 | 1 | 315 | 1 | 315 |
| QUR70357.1 | 3.27e-227 | 1 | 315 | 1 | 315 |
| ATD77577.1 | 3.27e-227 | 1 | 315 | 1 | 315 |
| VFA47692.1 | 3.27e-227 | 1 | 315 | 1 | 315 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5EKP_A | 5.07e-72 | 1 | 302 | 26 | 328 | Structureof the polyisoprenyl-phosphate glycosyltransferase GtrB (WT) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKP_B Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (WT) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKP_C Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (WT) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKP_D Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (WT) [Synechocystis sp. PCC 6803 substr. Kazusa] |
| 5EKE_A | 2.85e-71 | 1 | 302 | 26 | 328 | Structureof the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_B Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_C Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_D Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa] |
| 5MLZ_A | 3.95e-16 | 1 | 226 | 23 | 265 | Dolichylphosphate mannose synthase in complex with GDP and Mg2+ [Pyrococcus furiosus DSM 3638],5MM0_A Dolichyl phosphate mannose synthase in complex with GDP-mannose and Mn2+ (donor complex) [Pyrococcus furiosus DSM 3638],5MM1_A Dolichyl phosphate mannose synthase in complex with GDP and dolichyl phosphate mannose [Pyrococcus furiosus DSM 3638] |
| 4Y6N_A | 3.32e-07 | 3 | 166 | 49 | 202 | Crystalstructure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate-glucose (UDP-Glc) and phosphoglyceric acid (PGA) - GpgS Mn2+ UDP-Glc PGA-1 [Mycobacterium tuberculosis H37Rv],4Y6U_A Mycobacterial protein [Mycobacterium tuberculosis H37Rv],4Y7F_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate-glucose (UDP-Glc) and 3-(phosphonooxy)propanoic acid (PPA) - GpgS Mn2+ UDP-Glc PPA [Mycobacterium tuberculosis H37Rv],4Y7G_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate-glucose (UDP-Glc) and glycerol 3-phosphate (G3P) - GpgS Mn2+ UDP-Glc G3P [Mycobacterium tuberculosis H37Rv],4Y9X_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate-glucose (UDP-Glc) and phosphoglyceric acid (PGA) - GpgS Mn2+ UDP-Glc PGA-3 [Mycobacterium tuberculosis H37Rv],5JQX_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with phosphoglyceric acid (PGA) - GpgS*PGA [Mycobacterium tuberculosis H37Ra],5JQX_B Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with phosphoglyceric acid (PGA) - GpgS*PGA [Mycobacterium tuberculosis H37Ra],5JQX_C Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with phosphoglyceric acid (PGA) - GpgS*PGA [Mycobacterium tuberculosis H37Ra],5JQX_D Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with phosphoglyceric acid (PGA) - GpgS*PGA [Mycobacterium tuberculosis H37Ra],5JSX_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+ and uridine-diphosphate-glucose (UDP-Glc) [Mycobacterium tuberculosis H37Ra],5JT0_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate (UDP) and glucosyl-3-phosphoglycerate (GPG) - GpgS*GPG*UDP*Mn2+ [Mycobacterium tuberculosis H37Rv],5JUC_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with Mn2+, uridine-diphosphate (UDP) and glucosyl-3-phosphoglycerate (GPG) - GpgS*GPG*UDP*Mn2+_2 [Mycobacterium tuberculosis H37Rv],5JUD_A Crystal structure of glucosyl-3-phosphoglycerate synthase from Mycobacterium tuberculosis in complex with uridine-diphosphate (UDP) - GpgS*UDP [Mycobacterium tuberculosis variant bovis AF2122/97] |
| 3E25_A | 3.42e-07 | 3 | 166 | 45 | 198 | ChainA, Crystal structure of M. tuberculosis glucosyl-3-phosphoglycerate synthase [Mycobacterium tuberculosis],3E26_A Chain A, Crystal structure of M. tuberculosis glucosyl-3-phosphoglycerate synthase [Mycobacterium tuberculosis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P57022 | 8.59e-85 | 1 | 301 | 1 | 302 | Bactoprenol glucosyl transferase OS=Salmonella phage P22 OX=10754 GN=gtrB PE=3 SV=1 |
| P77293 | 1.73e-83 | 1 | 305 | 1 | 306 | Prophage bactoprenol glucosyl transferase homolog OS=Escherichia coli (strain K12) OX=83333 GN=yfdH PE=1 SV=1 |
| Q9T1D6 | 1.35e-82 | 1 | 301 | 1 | 302 | Bactoprenol glucosyl transferase OS=Shigella phage SfX OX=10874 GN=gtrB PE=3 SV=1 |
| P68667 | 6.13e-82 | 1 | 308 | 1 | 309 | SfII prophage-derived bactoprenol glucosyl transferase OS=Shigella flexneri OX=623 GN=gtrB PE=3 SV=1 |
| P68668 | 6.13e-82 | 1 | 308 | 1 | 309 | Bactoprenol glucosyl transferase OS=Shigella phage SfII OX=66284 GN=gtrB PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
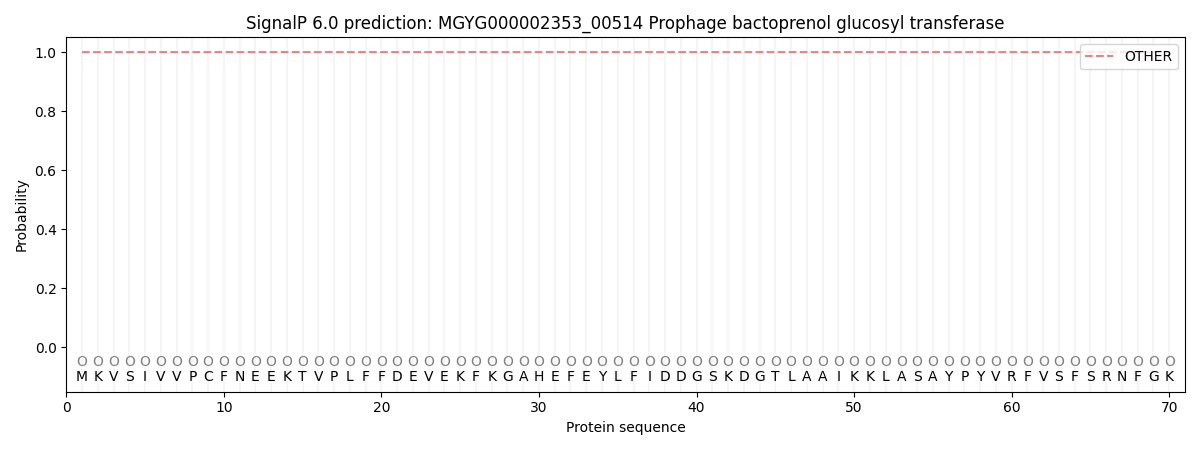
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000042 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |