You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002382_03653
You are here: Home > Sequence: MGYG000002382_03653
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Stenotrophomonas maltophilia_AK | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Gammaproteobacteria; Xanthomonadales; Xanthomonadaceae; Stenotrophomonas; Stenotrophomonas maltophilia_AK | |||||||||||
| CAZyme ID | MGYG000002382_03653 | |||||||||||
| CAZy Family | GH23 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 60667; End: 61257 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd13400 | LT_IagB-like | 1.21e-07 | 42 | 177 | 2 | 109 | Escherichia coli invasion protein IagB and similar proteins. Lytic transglycosylase-like protein, similar to Escherichia coli invasion protein IagB. IagB is encoded within a pathogenicity island in Salmonella enterica and has been shown to degrade polymeric peptidoglycan. IagB-like invasion proteins are implicated in the invasion of eukaryotic host cells by bacteria. Lytic transglycosylase (LT) catalyzes the cleavage of the beta-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetyl-D-glucosamine (GlcNAc), as do "goose-type" lysozymes. However, in addition to this, they also make a new glycosidic bond with the C6 hydroxyl group of the same muramic acid residue. Members of this family resemble the soluble and insoluble membrane-bound LTs in bacteria and the LTs in bacteriophage lambda. |
| pfam01464 | SLT | 6.74e-07 | 37 | 171 | 4 | 106 | Transglycosylase SLT domain. This family is distantly related to pfam00062. Members are found in phages, type II, type III and type IV secretion systems. |
| cd16892 | LT_VirB1-like | 1.09e-05 | 36 | 140 | 1 | 98 | VirB1-like subfamily. This subfamily includes VirB1 protein, one of twelve proteins making up type IV secretion systems (T4SS). T4SS are macromolecular assemblies generally composed of VirB1-11 and VirD4 proteins, and are used by bacteria to transport material across their membranes. VirB1 acts as a lytic transglycosylase (LT), and is important with respect to piercing the peptidoglycan layer in the periplasm. LTs catalyze the cleavage of the beta-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetyl-D-glucosamine (GlcNAc) as do "goose-type" lysozymes. However, in addition to this, they also make a new glycosidic bond with the C6 hydroxyl group of the same muramic acid residue. Proteins similar to this family include the soluble and insoluble membrane-bound LTs in bacteria, the LTs in bacteriophage lambda, as well as the eukaryotic "goose-type" lysozymes (goose egg-white lysozyme; GEWL). |
| COG0741 | MltE | 0.002 | 32 | 190 | 141 | 263 | Soluble lytic murein transglycosylase and related regulatory proteins (some contain LysM/invasin domains) [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AYW40245.1 | 9.11e-127 | 1 | 196 | 1 | 196 |
| QQK68050.1 | 1.29e-126 | 1 | 196 | 1 | 196 |
| BCN39848.1 | 1.29e-126 | 1 | 196 | 1 | 196 |
| AWQ06890.1 | 1.29e-126 | 1 | 196 | 1 | 196 |
| QOY21908.1 | 1.29e-126 | 1 | 196 | 1 | 196 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
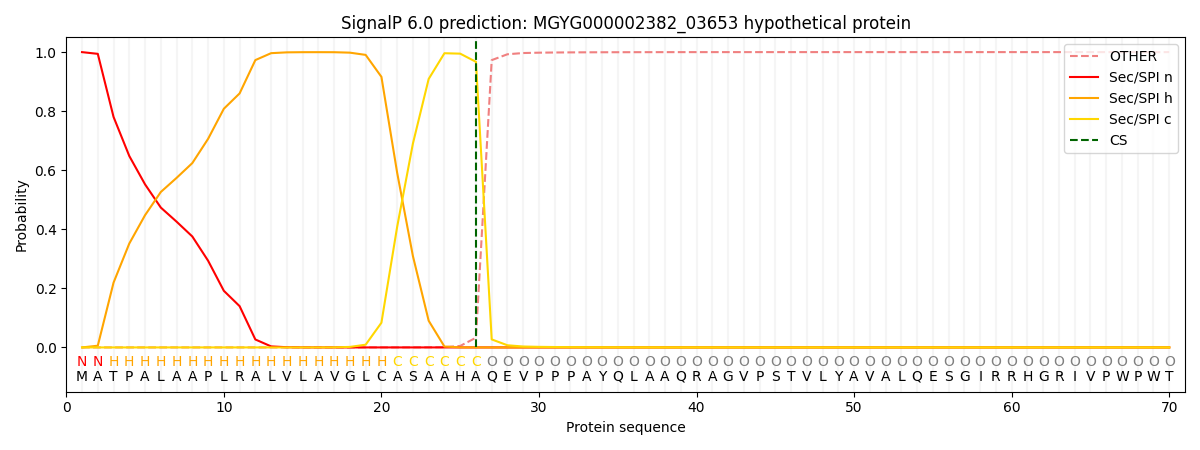
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000465 | 0.998621 | 0.000351 | 0.000213 | 0.000178 | 0.000154 |
