You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002403_04333
You are here: Home > Sequence: MGYG000002403_04333
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Robinsoniella peoriensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Robinsoniella; Robinsoniella peoriensis | |||||||||||
| CAZyme ID | MGYG000002403_04333 | |||||||||||
| CAZy Family | GH127 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 113015; End: 118129 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH127 | 341 | 546 | 3.4e-26 | 0.3893129770992366 |
| CBM66 | 1143 | 1282 | 1.6e-22 | 0.8516129032258064 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam07944 | Glyco_hydro_127 | 1.09e-43 | 110 | 546 | 62 | 502 | Beta-L-arabinofuranosidase, GH127. One member of this family, from Bidobacterium longicum, UniProtKB:E8MGH8, has been characterized as an unusual beta-L-arabinofuranosidase enzyme, EC:3.2.1.185. It rleases l-arabinose from the l-arabinofuranose (Araf)-beta1,2-Araf disaccharide and also transglycosylates 1-alkanols with retention of the anomeric configuration. Terminal beta-l-arabinofuranosyl residues have been found in arabinogalactan proteins from a mumber of different plantt species. beta-l-Arabinofuranosyl linkages with 1-4 arabinofuranosides are also found in the sugar chains of extensin and solanaceous lectins, hydroxyproline (Hyp)2-rich glycoproteins that are widely observed in plant cell wall fractions. The critical residue for catalytic activity is Glu-338, in a ET/SCAS sequence context. |
| COG3533 | COG3533 | 6.06e-18 | 306 | 563 | 270 | 509 | Uncharacterized conserved protein, DUF1680 family [Function unknown]. |
| pfam06439 | DUF1080 | 3.01e-08 | 1118 | 1285 | 9 | 182 | Domain of Unknown Function (DUF1080). This family has structural similarity to an endo-1,3-1,4-beta glucanase belonging to glycoside hydrolase family 16. However, the structure surrounding the active site differs from that of the endo-1,3-1,4-beta glucanase. |
| COG0810 | TonB | 4.09e-07 | 1468 | 1641 | 41 | 200 | Periplasmic protein TonB, links inner and outer membranes [Cell wall/membrane/envelope biogenesis]. |
| NF033909 | opacity_OapA | 8.71e-07 | 1468 | 1576 | 251 | 398 | opacity-associated protein OapA. This family consists of full-length homologs to OapA, opacity-associated protein A as described in Haemophilus influenzae. OapA shares a C-terminal homology domain, called the OapA domain, with the Escherichia coli protein YtfB, which is now known to bind peptidoglycan through its OapA domain and to act as a cell division protein. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QNF30112.1 | 1.41e-141 | 43 | 724 | 29 | 740 |
| ALL07583.1 | 4.43e-138 | 39 | 685 | 25 | 667 |
| QPH39723.1 | 2.05e-137 | 45 | 672 | 29 | 652 |
| AOM80627.1 | 1.25e-135 | 42 | 672 | 22 | 648 |
| QJE95537.1 | 2.81e-135 | 54 | 674 | 40 | 653 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
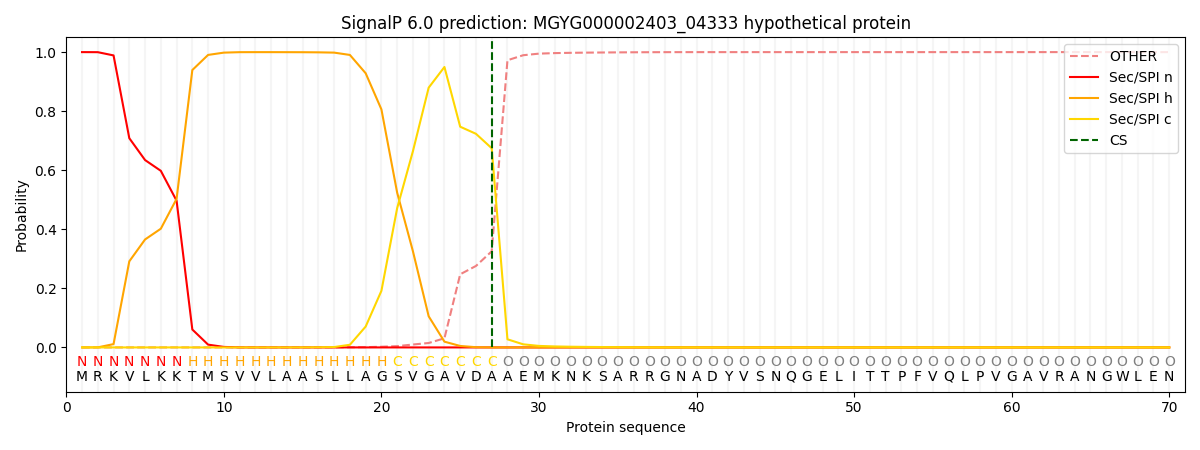
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000395 | 0.998699 | 0.000237 | 0.000249 | 0.000206 | 0.000176 |
