You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002473_01578
You are here: Home > Sequence: MGYG000002473_01578
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Victivallis vadensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Verrucomicrobiota; Lentisphaeria; Victivallales; Victivallaceae; Victivallis; Victivallis vadensis | |||||||||||
| CAZyme ID | MGYG000002473_01578 | |||||||||||
| CAZy Family | GH51 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 21036; End: 23063 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH51 | 21 | 360 | 5.5e-52 | 0.49206349206349204 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3534 | AbfA | 7.87e-31 | 23 | 360 | 6 | 352 | Alpha-L-arabinofuranosidase [Carbohydrate transport and metabolism]. |
| pfam01229 | Glyco_hydro_39 | 1.33e-06 | 181 | 358 | 151 | 310 | Glycosyl hydrolases family 39. |
| cd21510 | agarase_cat | 4.43e-05 | 144 | 260 | 76 | 187 | alpha-beta barrel catalytic domain of agarase, such as GH86-like endo-acting agarases identified in non-marine organisms. Typically, agarases (E.C. 3.2.1.81) are found in ocean-dwelling bacteria since agarose is a principle component of red algae cell wall polysaccharides. Agarose is a linear polymer of alternating D-galactose and 3,6-anhydro-L-galactopyranose. Endo-acting agarases, such as glycoside hydrolase 16 (GH16) and GH86 hydrolyze internal beta-1,4 linkages. GH86-like endo-acting agarase of this protein family has been identified in the human intestinal bacterium Bacteroides uniformis. This acquired metabolic pathway, as demonstrated by the prevalence of agar-specific genetic cluster called polysaccharide utilization loci (PULs), varies considerably between human populations, being much more prevalent in a Japanese sample than in North America, European, or Chinese samples. Agarase activity was also identified in the non-marine bacterium Cellvibrio sp. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AHF91758.1 | 7.09e-113 | 20 | 667 | 37 | 722 |
| AVM45703.1 | 5.90e-111 | 13 | 669 | 14 | 686 |
| AVM45928.1 | 2.82e-109 | 4 | 666 | 6 | 687 |
| AHF89202.1 | 1.38e-108 | 7 | 669 | 14 | 700 |
| SDT94913.1 | 3.92e-108 | 25 | 669 | 38 | 695 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3S2C_A | 2.59e-25 | 23 | 408 | 5 | 369 | Structureof the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_B Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_C Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_D Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_E Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_F Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_G Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_H Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_I Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_J Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_K Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_L Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1] |
| 4ATW_A | 3.40e-25 | 23 | 408 | 5 | 369 | Thecrystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_B The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_C The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_D The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_E The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_F The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8] |
| 3UG3_A | 4.10e-25 | 23 | 408 | 25 | 389 | Crystalstructure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG4_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG5_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima] |
| 2Y2W_A | 1.71e-19 | 13 | 325 | 45 | 352 | Elucidationof the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum],2Y2W_B Elucidation of the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum],2Y2W_C Elucidation of the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum],2Y2W_D Elucidation of the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum],2Y2W_E Elucidation of the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum],2Y2W_F Elucidation of the substrate specificity and protein structure of AbfB, a family 51 alpha-L-arabinofuranosidase from Bifidobacterium longum. [Bifidobacterium longum] |
| 5O7Z_A | 3.53e-16 | 23 | 412 | 5 | 421 | ChainA, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_B Chain B, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_C Chain C, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_D Chain D, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_E Chain E, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_F Chain F, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_A Chain A, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_B Chain B, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_C Chain C, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_D Chain D, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_E Chain E, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_F Chain F, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P53627 | 6.05e-22 | 24 | 323 | 7 | 303 | Intracellular exo-alpha-(1->5)-L-arabinofuranosidase OS=Streptomyces lividans OX=1916 GN=abfA PE=1 SV=1 |
| Q841V6 | 9.09e-19 | 13 | 325 | 45 | 352 | Intracellular exo-alpha-(1->5)-L-arabinofuranosidase OS=Bifidobacterium longum OX=216816 GN=abfB PE=1 SV=1 |
| E7CY70 | 3.03e-17 | 23 | 360 | 18 | 373 | Exo-alpha-(1->6)-L-arabinofuranosidase OS=Bifidobacterium longum OX=216816 GN=afuB PE=1 SV=1 |
| A3DIH0 | 3.58e-16 | 23 | 412 | 6 | 422 | Intracellular exo-alpha-(1->5)-L-arabinofuranosidase OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=Cthe_2548 PE=1 SV=1 |
| Q59219 | 1.93e-14 | 1 | 272 | 1 | 273 | Intracellular exo-alpha-L-arabinofuranosidase OS=Bacteroides ovatus OX=28116 GN=asdII PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
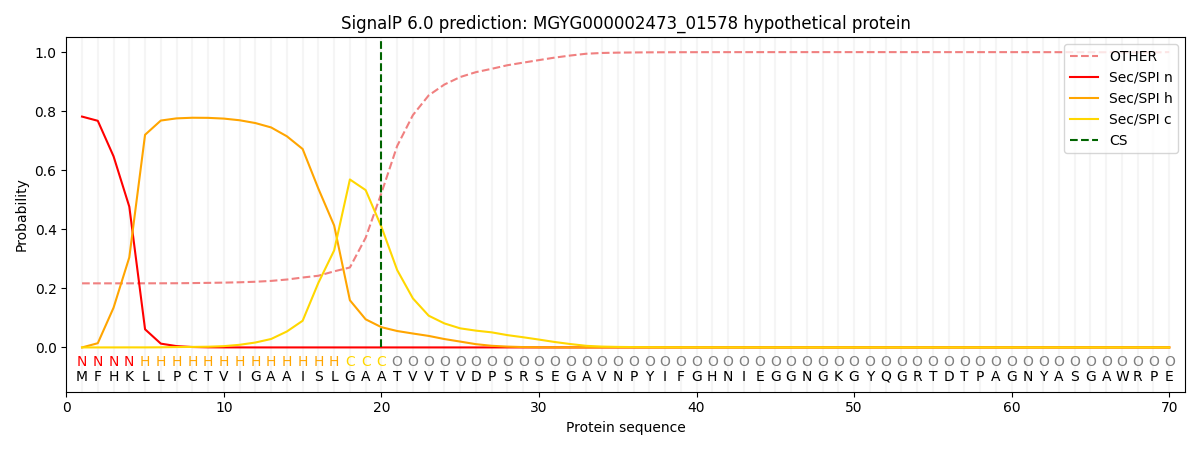
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.225392 | 0.771751 | 0.001914 | 0.000332 | 0.000285 | 0.000307 |
