You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002573_00994
You are here: Home > Sequence: MGYG000002573_00994
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Ruminococcus_C sp900545285 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus_C; Ruminococcus_C sp900545285 | |||||||||||
| CAZyme ID | MGYG000002573_00994 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 29091; End: 32669 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL9 | 610 | 1022 | 1.9e-122 | 0.9866666666666667 |
| PL1 | 237 | 407 | 1.2e-43 | 0.8217821782178217 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 2.93e-55 | 108 | 513 | 2 | 340 | Pectate lyase [Carbohydrate transport and metabolism]. |
| smart00656 | Amb_all | 2.18e-31 | 237 | 409 | 13 | 190 | Amb_all domain. |
| pfam00544 | Pec_lyase_C | 1.60e-22 | 243 | 405 | 38 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
| cd14256 | Dockerin_I | 1.07e-11 | 1126 | 1181 | 1 | 56 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| cd14253 | Dockerin | 1.60e-05 | 1127 | 1182 | 1 | 56 | Dockerin repeat domain. Dockerins are modules in the cellulosome complex that often anchor catalytic subunits by binding to cohesin domains of scaffolding proteins. Three types of dockerins and their corresponding cohesin have been described in the literature. This alignment models two consecutive dockerin repeats, the functional unit. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AOR96287.1 | 1.03e-273 | 14 | 1025 | 14 | 1084 |
| QMW93302.1 | 2.89e-273 | 14 | 1025 | 14 | 1084 |
| BBK78741.1 | 2.89e-273 | 14 | 1025 | 14 | 1084 |
| ADL51369.1 | 7.33e-268 | 2 | 1030 | 10 | 1247 |
| ABX41986.1 | 6.80e-265 | 43 | 1025 | 31 | 1154 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1RU4_A | 8.35e-38 | 614 | 925 | 18 | 326 | ChainA, Pectate lyase [Dickeya chrysanthemi] |
| 3VMV_A | 7.09e-23 | 194 | 423 | 27 | 259 | Crystalstructure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
| 1AIR_A | 5.28e-21 | 174 | 414 | 2 | 266 | ChainA, PECTATE LYASE C [Dickeya chrysanthemi],1O88_A Chain A, Pectate Lyase C [Dickeya chrysanthemi],1O8D_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8E_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8F_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8G_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8H_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8I_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8J_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8K_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8L_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8M_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1PLU_A Chain A, Protein (pectate Lyase C) [Dickeya chrysanthemi],2PEC_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi] |
| 2EWE_A | 1.28e-20 | 174 | 414 | 2 | 266 | ChainA, Pectate lyase C [Dickeya chrysanthemi] |
| 1VBL_A | 2.17e-15 | 232 | 405 | 124 | 330 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P0C1A6 | 1.51e-38 | 614 | 925 | 43 | 351 | Pectate lyase L OS=Dickeya chrysanthemi OX=556 GN=pelL PE=3 SV=1 |
| P0C1A7 | 7.50e-37 | 614 | 925 | 43 | 351 | Pectate lyase L OS=Dickeya dadantii (strain 3937) OX=198628 GN=pelL PE=1 SV=1 |
| P22751 | 3.55e-35 | 610 | 895 | 388 | 639 | Pectate disaccharide-lyase OS=Dickeya chrysanthemi OX=556 GN=pelX PE=1 SV=1 |
| Q8GCB2 | 3.40e-23 | 189 | 414 | 57 | 281 | Pectate trisaccharide-lyase OS=Bacillus licheniformis OX=1402 GN=pelA PE=1 SV=1 |
| Q65DC2 | 3.40e-23 | 189 | 414 | 57 | 281 | Pectate trisaccharide-lyase OS=Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46) OX=279010 GN=BLi04129 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
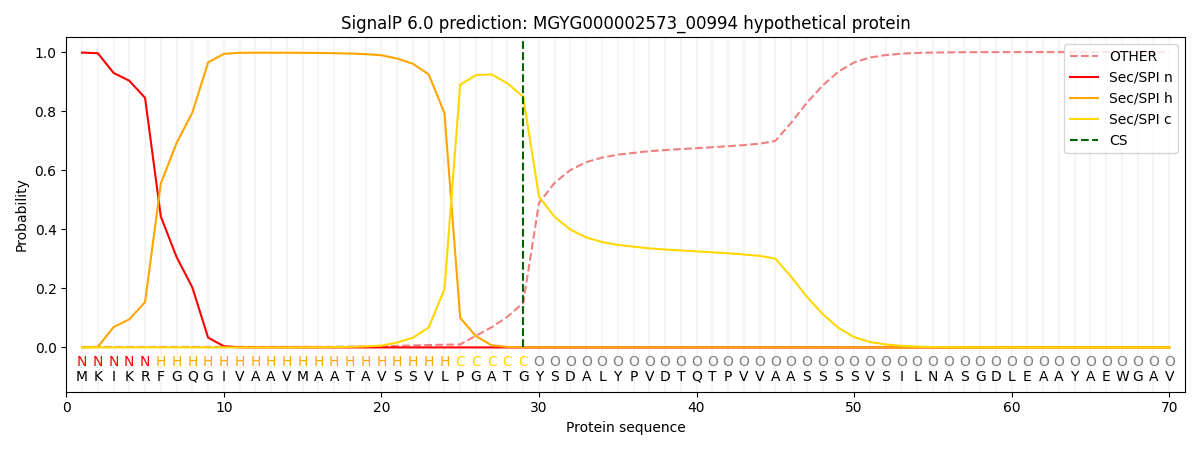
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002149 | 0.996740 | 0.000435 | 0.000270 | 0.000189 | 0.000162 |
