You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002603_01628
You are here: Home > Sequence: MGYG000002603_01628
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
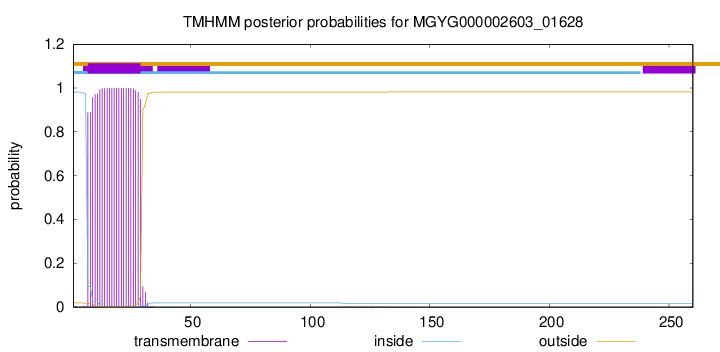
TMHMM annotations
Basic Information help
| Species | Prevotella sp900767615 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900767615 | |||||||||||
| CAZyme ID | MGYG000002603_01628 | |||||||||||
| CAZy Family | GH25 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 13073; End: 13855 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH25 | 65 | 240 | 5.7e-50 | 0.9943502824858758 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd00599 | GH25_muramidase | 1.70e-52 | 64 | 249 | 2 | 186 | Endo-N-acetylmuramidases (muramidases) are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. This family of muramidases contains a glycosyl hydrolase family 25 (GH25) catalytic domain and is found in bacteria, fungi, slime molds, round worms, protozoans and bacteriophages. The bacteriophage members are referred to as endolysins which are involved in lysing the host cell at the end of the replication cycle to allow release of mature phage particles. Endolysins are typically modular enzymes consisting of a catalytically active domain that hydrolyzes the peptidoglycan cell wall and a cell wall-binding domain that anchors the protein to the cell wall. Endolysins generally have narrow substrate specificities with either intra-species or intra-genus bacteriolytic activity. |
| cd06524 | GH25_YegX-like | 7.76e-52 | 64 | 249 | 2 | 192 | YegX is an uncharacterized bacterial protein with a glycosyl hydrolase family 25 (GH25) catalytic domain that is similar in sequence to the CH-type (Chalaropsis-type) lysozymes of the GH25 family of endolysins. |
| cd06413 | GH25_muramidase_1 | 3.16e-47 | 64 | 249 | 5 | 189 | Uncharacterized bacterial muramidase containing a glycosyl hydrolase family 25 (GH25) catalytic domain. Endo-N-acetylmuramidases are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. |
| cd06525 | GH25_Lyc-like | 2.99e-40 | 64 | 249 | 2 | 184 | Lyc muramidase is an autolytic lysozyme (autolysin) from Clostridium acetobutylicum encoded by the lyc gene. Lyc has a glycosyl hydrolase family 25 (GH25) catalytic domain. Endo-N-acetylmuramidases are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. |
| pfam01183 | Glyco_hydro_25 | 1.59e-33 | 65 | 240 | 1 | 180 | Glycosyl hydrolases family 25. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QNT66605.1 | 4.49e-186 | 1 | 260 | 1 | 260 |
| QUB71600.1 | 2.69e-75 | 58 | 260 | 30 | 231 |
| QUB85048.1 | 4.48e-75 | 62 | 260 | 39 | 236 |
| QUB85739.1 | 8.35e-73 | 62 | 260 | 39 | 236 |
| AKU70126.1 | 8.35e-73 | 62 | 260 | 39 | 236 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3HMC_A | 1.46e-13 | 65 | 256 | 8 | 186 | Endolysinfrom Bacillus anthracis [Bacillus anthracis] |
| 5A6S_A | 1.60e-11 | 66 | 247 | 26 | 199 | Crystalstructure of the CTP1L endolysin reveals how its activity is regulated by a secondary translation product [Clostridium phage phiCTP1] |
| 5JIP_A | 5.02e-07 | 64 | 247 | 16 | 220 | Crystalstructure of the Clostridium perfringens spore cortex lytic enzyme SleM [Clostridium perfringens],5JIP_B Crystal structure of the Clostridium perfringens spore cortex lytic enzyme SleM [Clostridium perfringens] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as OTHER
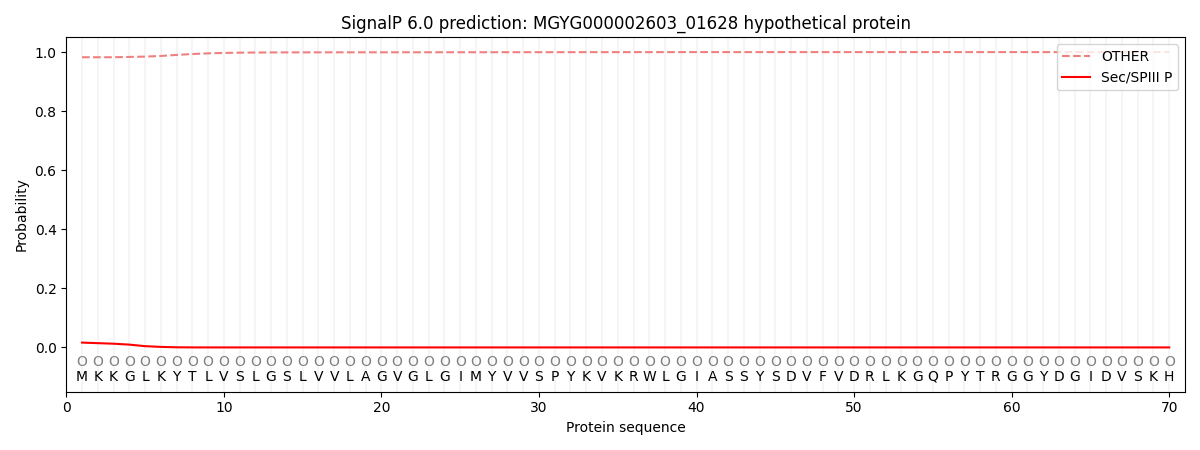
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.982624 | 0.000733 | 0.000296 | 0.000006 | 0.000003 | 0.016379 |