You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002701_00181
You are here: Home > Sequence: MGYG000002701_00181
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
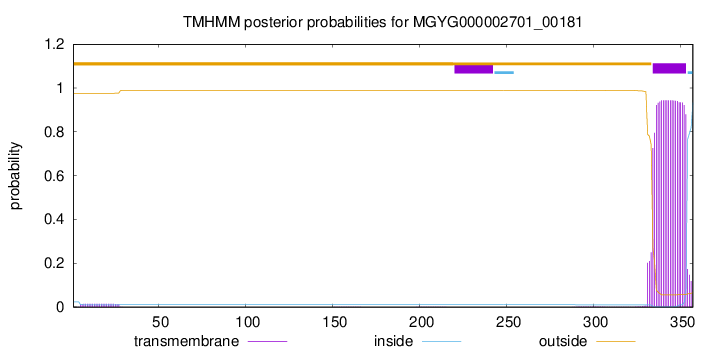
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia_A; Christensenellales; Christensenellaceae; QANA01; | |||||||||||
| CAZyme ID | MGYG000002701_00181 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | putative glycosyltransferase EpsJ | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 5883; End: 6956 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 7 | 129 | 6e-29 | 0.7294117647058823 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00535 | Glycos_transf_2 | 4.16e-31 | 7 | 174 | 1 | 164 | Glycosyl transferase family 2. Diverse family, transferring sugar from UDP-glucose, UDP-N-acetyl- galactosamine, GDP-mannose or CDP-abequose, to a range of substrates including cellulose, dolichol phosphate and teichoic acids. |
| cd04179 | DPM_DPG-synthase_like | 9.84e-26 | 9 | 118 | 2 | 114 | DPM_DPG-synthase_like is a member of the Glycosyltransferase 2 superfamily. DPM1 is the catalytic subunit of eukaryotic dolichol-phosphate mannose (DPM) synthase. DPM synthase is required for synthesis of the glycosylphosphatidylinositol (GPI) anchor, N-glycan precursor, protein O-mannose, and C-mannose. In higher eukaryotes,the enzyme has three subunits, DPM1, DPM2 and DPM3. DPM is synthesized from dolichol phosphate and GDP-Man on the cytosolic surface of the ER membrane by DPM synthase and then is flipped onto the luminal side and used as a donor substrate. In lower eukaryotes, such as Saccharomyces cerevisiae and Trypanosoma brucei, DPM synthase consists of a single component (Dpm1p and TbDpm1, respectively) that possesses one predicted transmembrane region near the C terminus for anchoring to the ER membrane. In contrast, the Dpm1 homologues of higher eukaryotes, namely fission yeast, fungi, and animals, have no transmembrane region, suggesting the existence of adapter molecules for membrane anchoring. This family also includes bacteria and archaea DPM1_like enzymes. However, the enzyme structure and mechanism of function are not well understood. The UDP-glucose:dolichyl-phosphate glucosyltransferase (DPG_synthase) is a transmembrane-bound enzyme of the endoplasmic reticulum involved in protein N-linked glycosylation. This enzyme catalyzes the transfer of glucose from UDP-glucose to dolichyl phosphate. This protein family belongs to Glycosyltransferase 2 superfamily. |
| PRK10073 | PRK10073 | 6.23e-24 | 1 | 179 | 1 | 181 | putative glycosyl transferase; Provisional |
| cd04187 | DPM1_like_bac | 4.33e-23 | 8 | 126 | 1 | 121 | Bacterial DPM1_like enzymes are related to eukaryotic DPM1. A family of bacterial enzymes related to eukaryotic DPM1; Although the mechanism of eukaryotic enzyme is well studied, the mechanism of the bacterial enzymes is not well understood. The eukaryotic DPM1 is the catalytic subunit of eukaryotic Dolichol-phosphate mannose (DPM) synthase. DPM synthase is required for synthesis of the glycosylphosphatidylinositol (GPI) anchor, N-glycan precursor, protein O-mannose, and C-mannose. The enzyme has three subunits, DPM1, DPM2 and DPM3. DPM is synthesized from dolichol phosphate and GDP-Man on the cytosolic surface of the ER membrane by DPM synthase and then is flipped onto the luminal side and used as a donor substrate. This protein family belongs to Glycosyltransferase 2 superfamily. |
| cd00761 | Glyco_tranf_GTA_type | 5.26e-22 | 9 | 118 | 2 | 113 | Glycosyltransferase family A (GT-A) includes diverse families of glycosyl transferases with a common GT-A type structural fold. Glycosyltransferases (GTs) are enzymes that synthesize oligosaccharides, polysaccharides, and glycoconjugates by transferring the sugar moiety from an activated nucleotide-sugar donor to an acceptor molecule, which may be a growing oligosaccharide, a lipid, or a protein. Based on the stereochemistry of the donor and acceptor molecules, GTs are classified as either retaining or inverting enzymes. To date, all GT structures adopt one of two possible folds, termed GT-A fold and GT-B fold. This hierarchy includes diverse families of glycosyl transferases with a common GT-A type structural fold, which has two tightly associated beta/alpha/beta domains that tend to form a continuous central sheet of at least eight beta-strands. The majority of the proteins in this superfamily are Glycosyltransferase family 2 (GT-2) proteins. But it also includes families GT-43, GT-6, GT-8, GT13 and GT-7; which are evolutionarily related to GT-2 and share structure similarities. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QBE97844.1 | 1.98e-115 | 1 | 357 | 1 | 358 |
| QIB54483.1 | 2.27e-114 | 1 | 357 | 1 | 358 |
| QMW78998.1 | 2.27e-114 | 1 | 357 | 1 | 358 |
| QEK17939.1 | 5.19e-91 | 6 | 357 | 3 | 354 |
| BCK85582.1 | 2.05e-39 | 2 | 278 | 1 | 269 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5HEA_A | 3.72e-17 | 2 | 96 | 3 | 97 | CgTstructure in hexamer [Streptococcus parasanguinis FW213],5HEA_B CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEA_C CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEC_A CgT structure in dimer [Streptococcus parasanguinis FW213],5HEC_B CgT structure in dimer [Streptococcus parasanguinis FW213] |
| 3BCV_A | 2.48e-13 | 4 | 96 | 5 | 97 | Crystalstructure of a putative glycosyltransferase from Bacteroides fragilis [Bacteroides fragilis NCTC 9343],3BCV_B Crystal structure of a putative glycosyltransferase from Bacteroides fragilis [Bacteroides fragilis NCTC 9343] |
| 5TZE_C | 4.75e-11 | 7 | 215 | 4 | 202 | Crystalstructure of S. aureus TarS in complex with UDP-GlcNAc [Staphylococcus aureus],5TZE_E Crystal structure of S. aureus TarS in complex with UDP-GlcNAc [Staphylococcus aureus],5TZI_C Crystal structure of S. aureus TarS 1-349 [Staphylococcus aureus],5TZJ_A Crystal structure of S. aureus TarS 1-349 in complex with UDP-GlcNAc [Staphylococcus aureus],5TZJ_C Crystal structure of S. aureus TarS 1-349 in complex with UDP-GlcNAc [Staphylococcus aureus],5TZK_C Crystal structure of S. aureus TarS 1-349 in complex with UDP [Staphylococcus aureus] |
| 5TZ8_A | 7.19e-11 | 7 | 215 | 4 | 202 | Crystalstructure of S. aureus TarS [Staphylococcus aureus],5TZ8_B Crystal structure of S. aureus TarS [Staphylococcus aureus],5TZ8_C Crystal structure of S. aureus TarS [Staphylococcus aureus] |
| 3L7I_A | 3.31e-09 | 6 | 107 | 4 | 107 | Structureof the Wall Teichoic Acid Polymerase TagF [Staphylococcus epidermidis RP62A],3L7I_B Structure of the Wall Teichoic Acid Polymerase TagF [Staphylococcus epidermidis RP62A],3L7I_C Structure of the Wall Teichoic Acid Polymerase TagF [Staphylococcus epidermidis RP62A],3L7I_D Structure of the Wall Teichoic Acid Polymerase TagF [Staphylococcus epidermidis RP62A] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P71059 | 1.12e-28 | 4 | 292 | 3 | 289 | Uncharacterized glycosyltransferase EpsJ OS=Bacillus subtilis (strain 168) OX=224308 GN=epsJ PE=2 SV=1 |
| P71057 | 1.79e-24 | 1 | 305 | 1 | 302 | Putative glycosyltransferase EpsH OS=Bacillus subtilis (strain 168) OX=224308 GN=epsH PE=2 SV=1 |
| A0A0H2UR96 | 2.79e-18 | 2 | 120 | 1 | 120 | Glycosyltransferase GlyG OS=Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334 / TIGR4) OX=170187 GN=glyG PE=1 SV=1 |
| Q92IF9 | 3.50e-17 | 1 | 140 | 290 | 430 | Uncharacterized glycosyltransferase RC0461 OS=Rickettsia conorii (strain ATCC VR-613 / Malish 7) OX=272944 GN=RC0461 PE=3 SV=1 |
| Q57022 | 7.63e-17 | 1 | 131 | 1 | 134 | Uncharacterized glycosyltransferase HI_0868 OS=Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) OX=71421 GN=HI_0868 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
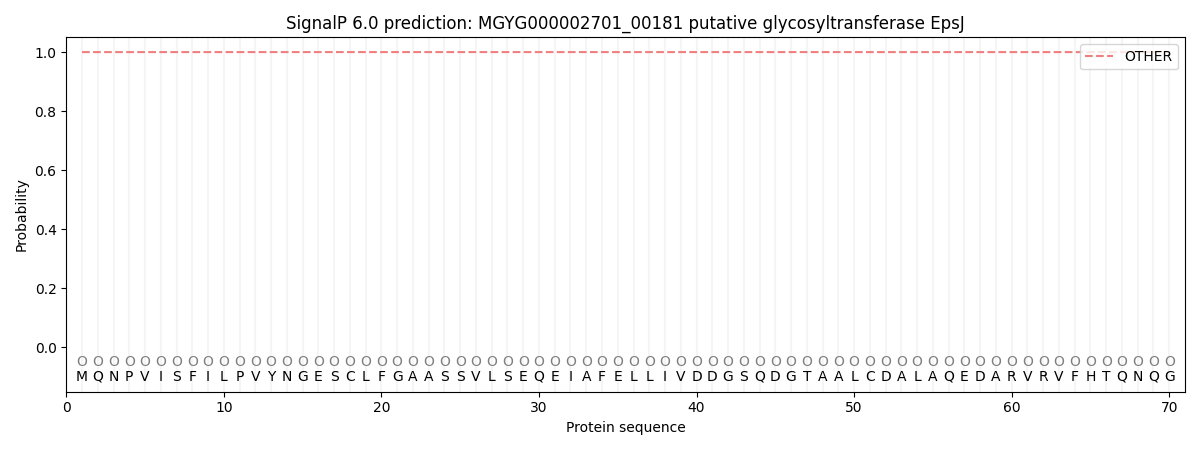
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000063 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |