You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002717_01233
You are here: Home > Sequence: MGYG000002717_01233
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; | |||||||||||
| CAZyme ID | MGYG000002717_01233 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 7569; End: 10118 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 525 | 770 | 8.8e-66 | 0.983739837398374 |
| GH28 | 70 | 427 | 5.8e-57 | 0.916923076923077 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18826 | GH43_CtGH43-like | 2.55e-145 | 515 | 769 | 1 | 259 | Glycosyl hydrolase family 43 protein similar to Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This uncharacterized glycosyl hydrolase family 43 (GH43) subgroup belongs to a subgroup which includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08985 | GH43_CtGH43-like | 5.65e-68 | 515 | 775 | 1 | 261 | Glycosyl hydrolase family 43 protein such as Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This glycosyl hydrolase family 43 (GH43) subgroup includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18825 | GH43_CtGH43-like | 2.98e-61 | 515 | 771 | 1 | 271 | Glycosyl hydrolase family 43 protein similar to Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This uncharacterized glycosyl hydrolase family 43 (GH43) subgroup belongs to a subgroup which includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| COG5434 | Pgu1 | 3.88e-60 | 63 | 353 | 102 | 399 | Polygalacturonase [Carbohydrate transport and metabolism]. |
| cd18821 | GH43_Pc3Gal43A-like | 7.50e-49 | 515 | 770 | 1 | 247 | Glycosyl hydrolase family 43 protein such as Phanerochaete chrysosporium exo-beta-1,3-galactanase (Pc1, 3Gal43A, 1,3Gal43A). This glycosyl hydrolase family 43 (GH43) subgroup includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), Fusarium oxysporum 12S Fo/1 (3Gal), and Streptomyces sp. 19(2012) SGalase1 and SGalase2. It belongs to the GH43_CtGH43 subgroup of the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43_CtGH43 includes proteins such as Clostridium thermocellum exo-beta-1,3-galactanase (Ct1,3Gal43A or CtGH43) which is comprised of the GH43 domain, a CBM13 domain, and a dockerin domain, exhibits an unusual ability to hydrolyze beta-1,3-galactan in the presence of a beta-1,6 linked branch, and is missing an essential acidic residue suggesting a mechanism by which it bypasses beta-1,6 linked branches in the substrate. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJR66962.1 | 0.0 | 1 | 849 | 1 | 849 |
| AII67852.1 | 0.0 | 1 | 849 | 1 | 849 |
| ALA73606.1 | 0.0 | 1 | 849 | 1 | 849 |
| AND19533.1 | 0.0 | 1 | 849 | 1 | 849 |
| QJR71302.1 | 0.0 | 1 | 849 | 1 | 849 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5OLP_A | 5.77e-57 | 30 | 447 | 26 | 440 | Galacturonidase[Bacteroides thetaiotaomicron VPI-5482],5OLP_B Galacturonidase [Bacteroides thetaiotaomicron VPI-5482] |
| 3JUR_A | 6.24e-52 | 60 | 448 | 44 | 434 | Thecrystal structure of a hyperthermoactive Exopolygalacturonase from Thermotoga maritima [Thermotoga maritima],3JUR_B The crystal structure of a hyperthermoactive Exopolygalacturonase from Thermotoga maritima [Thermotoga maritima],3JUR_C The crystal structure of a hyperthermoactive Exopolygalacturonase from Thermotoga maritima [Thermotoga maritima],3JUR_D The crystal structure of a hyperthermoactive Exopolygalacturonase from Thermotoga maritima [Thermotoga maritima] |
| 6EUG_A | 2.13e-42 | 493 | 846 | 21 | 374 | TheGH43, Beta 1,3 Galactosidase, BT3683 with galactoimidazole [Bacteroides thetaiotaomicron],6EUH_A The GH43, Beta 1,3 Galactosidase, BT3683 with galactodeoxynojirimycin [Bacteroides thetaiotaomicron VPI-5482],6EUH_B The GH43, Beta 1,3 Galactosidase, BT3683 with galactodeoxynojirimycin [Bacteroides thetaiotaomicron VPI-5482],6EUH_C The GH43, Beta 1,3 Galactosidase, BT3683 with galactodeoxynojirimycin [Bacteroides thetaiotaomicron VPI-5482],6EUI_A The GH43, Beta 1,3 Galactosidase, BT3683 with galactose [Bacteroides thetaiotaomicron VPI-5482] |
| 6EUF_A | 2.98e-35 | 501 | 845 | 5 | 302 | TheGH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUF_B The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUF_C The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUF_D The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUJ_A The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUJ_B The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUJ_C The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482],6EUJ_D The GH43, Beta 1,3 Galactosidase, BT0265 [Bacteroides thetaiotaomicron VPI-5482] |
| 3VSF_A | 3.22e-28 | 504 | 768 | 45 | 310 | ChainA, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSF_B Chain B, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSF_C Chain C, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSF_D Chain D, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSF_E Chain E, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSF_F Chain F, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_A Chain A, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_B Chain B, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_C Chain C, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_D Chain D, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_E Chain E, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VSZ_F Chain F, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_A Chain A, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_B Chain B, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_C Chain C, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_D Chain D, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_E Chain E, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT0_F Chain F, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_A Chain A, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_B Chain B, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_C Chain C, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_D Chain D, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_E Chain E, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT1_F Chain F, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_A Chain A, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_B Chain B, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_C Chain C, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_D Chain D, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_E Chain E, Ricin B lectin [Acetivibrio thermocellus ATCC 27405],3VT2_F Chain F, Ricin B lectin [Acetivibrio thermocellus ATCC 27405] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P27644 | 6.33e-25 | 199 | 413 | 28 | 249 | Polygalacturonase OS=Rhizobium radiobacter OX=358 GN=pgl PE=2 SV=1 |
| A7PZL3 | 7.55e-24 | 58 | 422 | 77 | 422 | Probable polygalacturonase OS=Vitis vinifera OX=29760 GN=GSVIVT00026920001 PE=1 SV=1 |
| P15922 | 2.11e-18 | 58 | 423 | 166 | 553 | Exo-poly-alpha-D-galacturonosidase OS=Dickeya chrysanthemi OX=556 GN=pehX PE=1 SV=1 |
| Q9LW07 | 1.36e-06 | 201 | 343 | 146 | 281 | Probable polygalacturonase At3g15720 OS=Arabidopsis thaliana OX=3702 GN=At3g15720 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
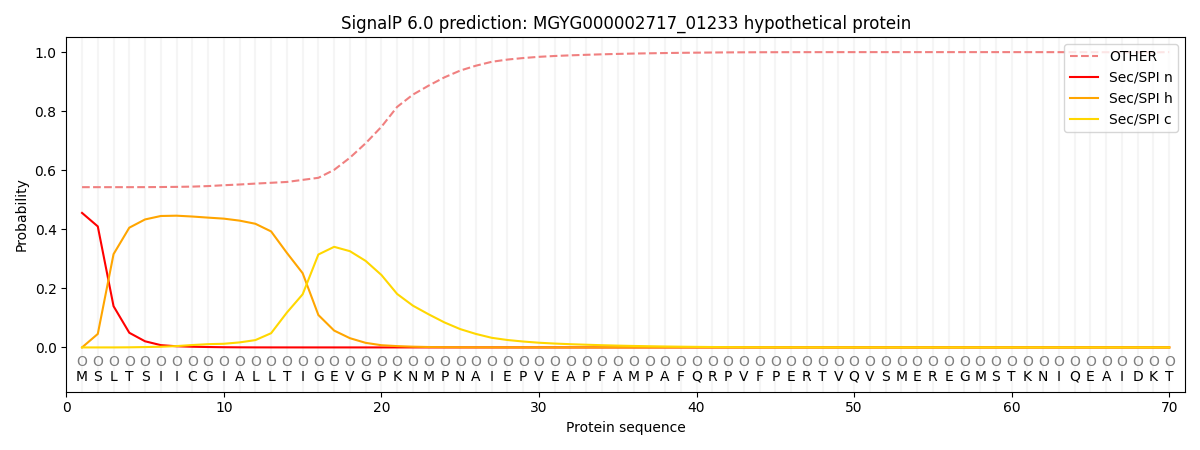
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.553020 | 0.443963 | 0.002359 | 0.000252 | 0.000180 | 0.000220 |
