You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002817_04546
You are here: Home > Sequence: MGYG000002817_04546
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Paenibacillus_B sp900539405 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Paenibacillales; Paenibacillaceae; Paenibacillus_B; Paenibacillus_B sp900539405 | |||||||||||
| CAZyme ID | MGYG000002817_04546 | |||||||||||
| CAZy Family | CBM13 | |||||||||||
| CAZyme Description | 1-phosphatidylinositol phosphodiesterase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 6493; End: 7548 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd08586 | PI-PLCc_BcPLC_like | 1.49e-92 | 44 | 346 | 1 | 279 | Catalytic domain of Bacillus cereus phosphatidylinositol-specific phospholipases C and similar proteins. This subfamily corresponds to the catalytic domain present in Bacillus cereus phosphatidylinositol-specific phospholipase C (PI-PLC, EC 4.6.1.13) and its sequence homologs found in bacteria and eukaryota. Bacterial PI-PLCs participate in Ca2+-independent PI metabolism, hydrolyzing the membrane lipid phosphatidylinositol (PI) to produce phosphorylated myo-inositol and diacylglycerol (DAG). Although their precise physiological function remains unclear, bacterial PI-PLCs may function as virulence factors in some pathogenic bacteria. Bacterial PI-PLCs contain a single TIM-barrel type catalytic domain. Their catalytic mechanism is based on general base and acid catalysis utilizing two well conserved histidines, and consists of two steps, a phosphotransfer and a phosphodiesterase reaction. This family also includes some uncharacterized eukaryotic homologs, which contains a single TIM-barrel type catalytic domain, X domain. They are similar to bacterial PI-PLCs, and distinct from typical eukaryotic PI-PLCs, which have a multidomain organization that consists of a PLC catalytic core domain, and various regulatory domains, and strictly require Ca2+ for their catalytic activities. The prototype of this family is Bacillus cereus PI-PLC, which has a moderate thermal stability and is active as a monomer. |
| cd08557 | PI-PLCc_bacteria_like | 1.66e-55 | 47 | 346 | 3 | 271 | Catalytic domain of bacterial phosphatidylinositol-specific phospholipase C and similar proteins. This subfamily corresponds to the catalytic domain present in bacterial phosphatidylinositol-specific phospholipase C (PI-PLC, EC 4.6.1.13) and their sequence homologs found in eukaryota. Bacterial PI-PLCs participate in Ca2+-independent PI metabolism, hydrolyzing the membrane lipid phosphatidylinositol (PI) to produce phosphorylated myo-inositol and diacylglycerol (DAG). Although their precise physiological function remains unclear, bacterial PI-PLCs may function as virulence factors in some pathogenic bacteria. Bacterial PI-PLCs contain a single TIM-barrel type catalytic domain. Its catalytic mechanism is based on general base and acid catalysis utilizing two well conserved histidines, and consists of two steps, a phosphotransfer and a phosphodiesterase reaction. Eukaryotic homologs in this family are named as phosphatidylinositol-specific phospholipase C X domain containing proteins (PI-PLCXD). They are distinct from the typical eukaryotic phosphoinositide-specific phospholipases C (PI-PLC, EC 3.1.4.11), which have a multidomain organization that consists of a PLC catalytic core domain, and various regulatory domains. The catalytic core domain is assembled from two highly conserved X- and Y-regions split by a divergent linker sequence. In contrast, eukaryotic PI-PLCXDs contain a single TIM-barrel type catalytic domain, X domain, which is closely related to that of bacterial PI-PLCs. Although the biological function of eukaryotic PI-PLCXDs still remains unclear, it may be distinct from that of typical eukaryotic PI-PLCs. This family also includes a distinctly different type of eukaryotic PLC, glycosylphosphatidylinositol-specific phospholipase C (GPI-PLC), an integral membrane protein characterized in the protozoan parasite Trypanosoma brucei. T. brucei GPI-PLC hydrolyzes the GPI-anchor on the variant specific glycoprotein (VSG), releasing dimyristyl glycerol (DMG), which may facilitate the evasion of the protozoan to the host's immune system. It does not require Ca2+ for its activity and is more closely related to bacterial PI-PLCs, but not mammalian PI-PLCs. |
| cd00137 | PI-PLCc | 2.28e-36 | 47 | 270 | 2 | 239 | Catalytic domain of prokaryotic and eukaryotic phosphoinositide-specific phospholipase C. This subfamily corresponds to the catalytic domain present in prokaryotic and eukaryotic phosphoinositide-specific phospholipase C (PI-PLC), which is a ubiquitous enzyme catalyzing the cleavage of the sn3-phosphodiester bond in the membrane phosphoinositides (phosphatidylinositol, PI; Phosphatidylinositol-4-phosphate, PIP; phosphatidylinositol 4,5-bisphosphate, PIP2) to yield inositol phosphates (inositol monosphosphate, InsP; inositol diphosphate, InsP2; inositol trisphosphate, InsP3) and diacylglycerol (DAG). The higher eukaryotic PI-PLCs (EC 3.1.4.11) have a multidomain organization that consists of a PLC catalytic core domain, and various regulatory domains. They play a critical role in most signal transduction pathways, controlling numerous cellular events, such as cell growth, proliferation, excitation and secretion. These PI-PLCs strictly require Ca2+ for their catalytic activity. They display a clear preference towards the hydrolysis of the more highly phosphorylated PI-analogues, PIP2 and PIP, to generate two important second messengers, InsP3 and DAG. InsP3 triggers inflow of calcium from intracellular stores, while DAG, together with calcium, activates protein kinase C, which then phosphorylates other molecules, leading to altered cellular activity. In contrast, bacterial PI-PLCs contain a single catalytic domain. Although their precise physiological function remains unclear, bacterial PI-PLCs may function as virulence factors in some pathogenic bacteria. They participate in Ca2+-independent PI metabolism. They are characterized as phosphatidylinositol-specific phospholipase C (EC 4.6.1.13) that selectively hydrolyze PI, not PIP or PIP2. The TIM-barrel type catalytic domain in bacterial PI-PLCs is very similar to the one in eukaryotic PI-PLCs, in which the catalytic domain is assembled from two highly conserved X- and Y-regions split by a divergent linker sequence. The catalytic mechanism of both prokaryotic and eukaryotic PI-PLCs is based on general base and acid catalysis utilizing two well conserved histidines, and consists of two steps, a phosphotransfer and a phosphodiesterase reaction. This superfamily also includes a distinctly different type of eukaryotic PLC, glycosylphosphatidylinositol-specific phospholipase C (GPI-PLC), an integral membrane protein characterized in the protozoan parasite Trypanosoma brucei. T. brucei GPI-PLC hydrolyzes the GPI-anchor on the variant specific glycoprotein (VSG), releasing dimyristyl glycerol (DMG), which may facilitate the evasion of the protozoan to the host#s immune system. It does not require Ca2+ for its activity and is more closely related to bacterial PI-PLCs, but not mammalian PI-PLCs. |
| cd08587 | PI-PLCXDc_like | 2.68e-20 | 50 | 232 | 6 | 212 | Catalytic domain of phosphatidylinositol-specific phospholipase C X domain containing and similar proteins. This family corresponds to the catalytic domain present in phosphatidylinositol-specific phospholipase C X domain containing proteins (PI-PLCXD) which are bacterial phosphatidylinositol-specific phospholipase C (PI-PLC, EC 4.6.1.13) sequence homologs mainly found in eukaryota. The typical eukaryotic phosphoinositide-specific phospholipase C (PI-PLC, EC 3.1.4.11) have a multidomain organization that consists of a PLC catalytic core domain, and various regulatory domains. The catalytic core domain is assembled from two highly conserved X- and Y-regions split by a divergent linker sequence. In contrast, eukaryotic PI-PLCXDs and their bacterial homologs contain a single TIM-barrel type catalytic domain, X domain, which is more closely related to that of bacterial PI-PLCs. Although the biological function of eukaryotic PI-PLCXDs still remains unclear, it may be distinct from that of typical eukaryotic PI-PLCs. |
| smart00148 | PLCXc | 1.07e-17 | 49 | 193 | 1 | 142 | Phospholipase C, catalytic domain (part); domain X. Phosphoinositide-specific phospholipases C. These enzymes contain 2 regions (X and Y) which together form a TIM barrel-like structure containing the active site residues. Phospholipase C enzymes (PI-PLC) act as signal transducers that generate two second messengers, inositol-1,4,5-trisphosphate and diacylglycerol. The bacterial enzyme appears to be a homologue of the mammalian PLCs. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QWS48512.1 | 2.71e-164 | 1 | 351 | 1 | 347 |
| AMR88637.1 | 2.71e-164 | 1 | 351 | 1 | 347 |
| AVF24765.1 | 1.73e-145 | 36 | 349 | 13 | 323 |
| AVF29525.1 | 1.73e-145 | 36 | 349 | 13 | 323 |
| AQZ47425.1 | 1.73e-145 | 36 | 349 | 13 | 323 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2PLC_A | 8.83e-31 | 43 | 347 | 6 | 273 | Phosphatidylinositol-SpecificPhospholipase C From Listeria Monocytogenes [Listeria monocytogenes] |
| 1AOD_A | 1.22e-30 | 43 | 347 | 20 | 287 | Phosphatidylinositol-SpecificPhospholipase C From Listeria Monocytogenes [Listeria monocytogenes] |
| 4S3G_A | 8.16e-28 | 42 | 286 | 10 | 277 | ChainA, 1-phosphatidylinositol phosphodiesterase [Staphylococcus aureus subsp. aureus str. Newman] |
| 3V16_A | 1.15e-27 | 42 | 286 | 10 | 277 | ChainA, 1-phosphatidylinositol phosphodiesterase [Staphylococcus aureus subsp. aureus str. Newman] |
| 3V18_A | 1.17e-27 | 42 | 286 | 9 | 276 | ChainA, 1-phosphatidylinositol phosphodiesterase [Staphylococcus aureus subsp. aureus str. Newman] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P34024 | 1.18e-29 | 43 | 347 | 48 | 315 | 1-phosphatidylinositol phosphodiesterase OS=Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) OX=169963 GN=plcA PE=1 SV=1 |
| Q2G1Q2 | 1.02e-27 | 1 | 286 | 1 | 303 | 1-phosphatidylinositol phosphodiesterase OS=Staphylococcus aureus (strain NCTC 8325 / PS 47) OX=93061 GN=plc PE=1 SV=1 |
| P45723 | 2.02e-27 | 26 | 286 | 5 | 287 | 1-phosphatidylinositol phosphodiesterase OS=Staphylococcus aureus (strain Newman) OX=426430 GN=plc PE=1 SV=2 |
| P08954 | 1.21e-23 | 43 | 286 | 44 | 301 | 1-phosphatidylinositol phosphodiesterase OS=Bacillus thuringiensis OX=1428 PE=1 SV=1 |
| P14262 | 4.36e-23 | 43 | 265 | 44 | 281 | 1-phosphatidylinositol phosphodiesterase OS=Bacillus cereus OX=1396 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
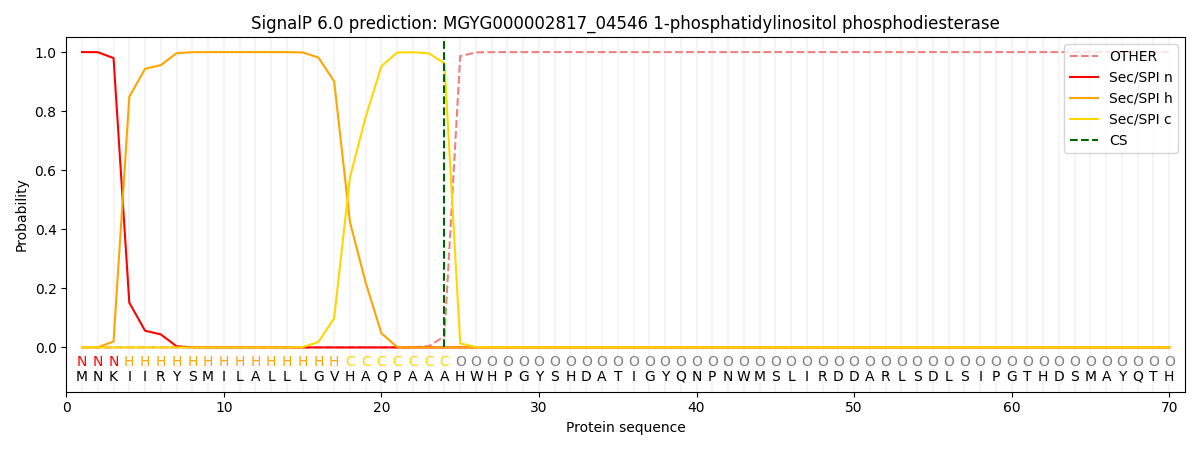
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000289 | 0.998919 | 0.000244 | 0.000194 | 0.000183 | 0.000177 |
