You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003072_00078
You are here: Home > Sequence: MGYG000003072_00078
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Paenibacillus amylolyticus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Paenibacillales; Paenibacillaceae; Paenibacillus; Paenibacillus amylolyticus | |||||||||||
| CAZyme ID | MGYG000003072_00078 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 75376; End: 76440 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM36 | 240 | 353 | 7.3e-53 | 0.991304347826087 |
| CE4 | 36 | 153 | 2.5e-32 | 0.8923076923076924 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10953 | CE4_SlAXE_like | 2.85e-95 | 38 | 215 | 1 | 179 | Catalytic NodB homology domain of Streptomyces lividans acetylxylan esterase and its bacterial homologs. This family is represented by Streptomyces lividans acetylxylan esterase (SlAXE, EC 3.1.1.72), a member of the carbohydrate esterase 4 (CE4) superfamily. SlAXE deacetylates O-acetylated xylan, a key component of plant cell walls. It shows no detectable activity on generic esterase substrates including para-nitrophenyl acetate. It is specific for sugar-based substrates and will precipitate acetylxylan as a result of deacetylation. SlAXE also functions as a chitin and chitooligosaccharide de-N-acetylase with equal efficiency to its activity on xylan. SlAXE forms a dimer. Each monomer contains a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold as other CE4 esterases, which encompasses a mononuclear metalloenzyme employing a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine), to carry out acid/base catalysis. SlAXE possess a single metal center with a chemical preference for Co2+. |
| cd10917 | CE4_NodB_like_6s_7s | 3.17e-71 | 38 | 206 | 1 | 171 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| cd04078 | CBM36_xylanase-like | 4.14e-65 | 237 | 353 | 2 | 118 | Carbohydrate Binding Module family 36 (CBM36); appended mainly to glycoside hydrolase family 11 (GH11) domains; xylan binding. This family includes carbohydrate binding module family 36 (CBM36) most of which appear appended to glycoside hydrolase family 11 (GH11) domains. These CBMs are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH11 catalytic modules with their dedicated, insoluble substrates. GH11 domains have xylanase (endo-1,4-beta-xylanase) activity which catalyzes the hydrolysis of beta-1,4 bonds of xylan, the major component of hemicelluloses, to generate xylooligosaccharides and xylose. This family includes XynB from Dictyoglomus thermophilum Rt46B.1 and Xyn11A from Pseudobutyrivibrio xylanivorans Mz5T. Xyn11A is a multicatalytic enzyme with an N-terminal GH11 domain, a CBM36 domain, and a C-terminal putative NodB-like polysaccharide deacetylase which is predicted to be an acetyl esterase involved in debranching activity in the xylan backbone. CBM6 is an unusual CBM as it represents a chimera of two distinct binding sites with different modes of binding: binding site I within the loop regions and binding site II on the concave face of the beta-sandwich fold. Consistent with its structural and sequence similarity to CBM6, CBM36 binds xylan, but only at binding site I, and in a calcium-dependent manner; the latter suggests its potential application in affinity labeling. |
| cd10954 | CE4_CtAXE_like | 1.07e-63 | 39 | 209 | 2 | 171 | Catalytic NodB homology domain of Clostridium thermocellum acetylxylan esterase and its bacterial homologs. This family is represented by Clostridium thermocellum acetylxylan esterase (CtAXE, EC 3.1.1.72), a member of the carbohydrate esterase 4 (CE4) superfamily. CtAXE deacetylates O-acetylated xylan, a key component of plant cell walls. It shows no detectable activity on generic esterase substrates including para-nitrophenyl acetate. It is specific for sugar-based substrates and will precipitate acetylxylan, as a consequence of deacetylation. CtAXE is a monomeric protein containing a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold as other CE4 esterases. However, due to differences in the topography of the substrate-binding groove, the chemistry of the active center, and metal ion coordination, CtAXE has different metal ion preference and lacks activity on N-acetyl substrates. It is significantly activated by Co2+. Moreover, CtAXE displays distinctly different ligand coordination to the metal ion, utilizing an aspartate, a histidine, and four water molecules, as opposed to the conserved His-His-Asp zinc-binding triad of other CE4 esterases. |
| cd10947 | CE4_SpPgdA_BsYjeA_like | 3.76e-54 | 39 | 209 | 2 | 171 | Catalytic NodB homology domain of Streptococcus pneumoniae peptidoglycan deacetylase PgdA, Bacillus subtilis BsYjeA protein, and their bacterial homologs. This family is represented by Streptococcus pneumoniae peptidoglycan GlcNAc deacetylase (SpPgdA), a member of the carbohydrate esterase 4 (CE4) superfamily. SpPgdA protects gram-positive bacterial cell wall from host lysozymes by deacetylating peptidoglycan N-acetylglucosamine (GlcNAc) residues. It consists of three separate domains: N-terminal, middle and C-terminal (catalytic) domains. The catalytic NodB homology domain is similar to the deformed (beta/alpha)8 barrel fold adopted by other CE4 esterases, which harbors a mononuclear metalloenzyme employing a conserved His-His-Asp zinc-binding triad closely associated with conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. The enzyme is able to accept GlcNAc3 as a substrate, with the N-acetyl of the middle sugar being removed by the enzyme. This family also includes Bacillus subtilis BsYjeA protein encoded by the yjeA gene, which is one of the six polysaccharide deacetylase gene homologs (pdaA, pdaB/ybaN, yheN, yjeA, yxkH and ylxY) in the Bacillus subtilis genome. Although homology comparison shows that the BsYjeA protein contains a polysaccharide deacetylase domain, and was predicted to be a membrane-bound xylanase or a membrane-bound chitooligosaccharide deacetylase, more recent research indicates BsYjeA might be a novel non-specific secretory endonuclease which creates random nicks progressively on the two strands of dsDNA, resulting in highly distinguishable intermediates/products very different in chemical and physical compositions over time. In addition, BsYjeA shares several enzymatic properties with the well-understood DNase I endonuclease. Both enzymes are active on ssDNA and dsDNA, both generate random nicks, and both require Mg2+ or Mn2+ for hydrolytic activity. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| APO45359.1 | 1.32e-248 | 1 | 354 | 1 | 354 |
| QZN73725.1 | 2.44e-244 | 1 | 354 | 1 | 354 |
| QLG41225.1 | 1.41e-227 | 1 | 354 | 1 | 354 |
| QOS78519.1 | 4.72e-226 | 1 | 354 | 1 | 354 |
| QKS55139.1 | 8.06e-211 | 1 | 353 | 1 | 353 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2CC0_A | 7.12e-84 | 33 | 228 | 1 | 195 | Family4 carbohydrate esterase from Streptomyces lividans in complex with acetate [Streptomyces lividans],2CC0_B Family 4 carbohydrate esterase from Streptomyces lividans in complex with acetate [Streptomyces lividans] |
| 7AY3_A | 1.11e-55 | 235 | 353 | 2 | 120 | Crystalstructure of the CBM36-1 domain of a multidomain xylanase from the hindgut metagenome of Trinervitermes trinervoides [uncultured bacterium] |
| 6KKA_A | 1.01e-52 | 237 | 353 | 210 | 326 | XylanaseJ mutant from Bacillus sp. 41M-1 [Bacillus sp. 41M-1],6KKA_B Xylanase J mutant from Bacillus sp. 41M-1 [Bacillus sp. 41M-1] |
| 6KJL_A | 1.04e-52 | 237 | 353 | 211 | 327 | XylanaseJ from Bacillus sp. strain 41M-1 [Bacillus sp. 41M-1],6KJL_B Xylanase J from Bacillus sp. strain 41M-1 [Bacillus sp. 41M-1] |
| 2DCJ_A | 2.03e-52 | 237 | 353 | 237 | 353 | Atwo-domain structure of alkaliphilic XynJ from Bacillus sp. 41M-1 [Bacillus sp. 41M-1],2DCJ_B A two-domain structure of alkaliphilic XynJ from Bacillus sp. 41M-1 [Bacillus sp. 41M-1],2DCK_A A tetragonal-lattice structure of alkaliphilic XynJ from Bacillus sp. 41M-1 [Bacillus sp. 41M-1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P54865 | 1.62e-63 | 34 | 225 | 352 | 544 | Bifunctional xylanase/deacetylase OS=Cellulomonas fimi OX=1708 GN=xynD PE=1 SV=1 |
| Q59674 | 2.32e-51 | 37 | 225 | 397 | 586 | Bifunctional xylanase/xylan deacetylase OS=Cellvibrio japonicus OX=155077 GN=xyn11A PE=1 SV=1 |
| P83513 | 2.79e-51 | 238 | 350 | 250 | 362 | Bifunctional xylanase/deacetylase OS=Pseudobutyrivibrio xylanivorans OX=185007 GN=xyn11A PE=1 SV=2 |
| P45796 | 3.61e-39 | 237 | 352 | 517 | 632 | Arabinoxylan arabinofuranohydrolase OS=Paenibacillus polymyxa OX=1406 GN=xynD PE=1 SV=1 |
| Q8DP63 | 1.49e-28 | 40 | 209 | 270 | 438 | Peptidoglycan-N-acetylglucosamine deacetylase OS=Streptococcus pneumoniae (strain ATCC BAA-255 / R6) OX=171101 GN=pgdA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
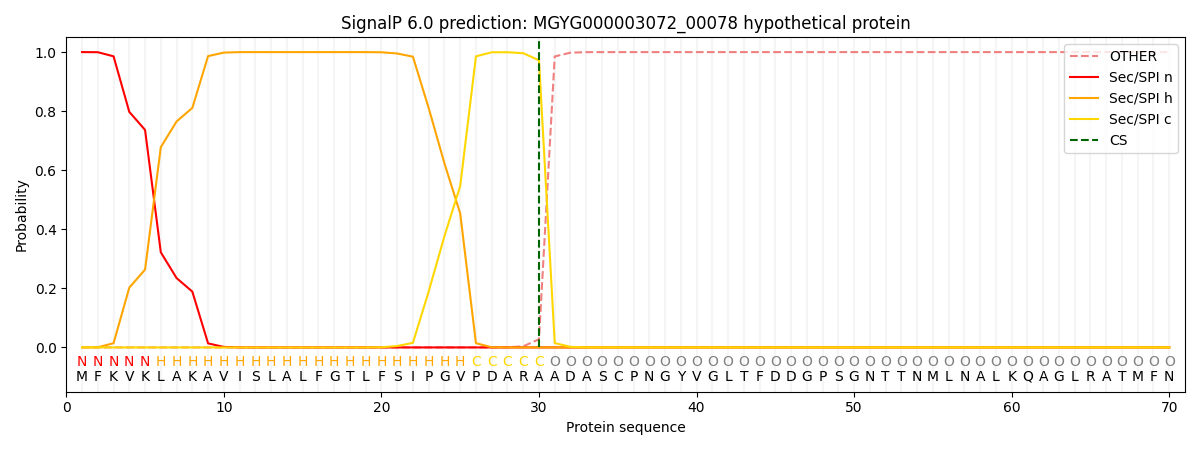
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000221 | 0.999140 | 0.000159 | 0.000178 | 0.000158 | 0.000143 |
