You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003132_00304
You are here: Home > Sequence: MGYG000003132_00304
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Dysgonomonas sp900556485 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Dysgonomonadaceae; Dysgonomonas; Dysgonomonas sp900556485 | |||||||||||
| CAZyme ID | MGYG000003132_00304 | |||||||||||
| CAZy Family | CBM50 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 334144; End: 335571 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd01825 | SGNH_hydrolase_peri1 | 1.31e-42 | 311 | 467 | 24 | 189 | SGNH_peri1; putative periplasmic member of the SGNH-family of hydrolases, a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| pfam01476 | LysM | 4.76e-13 | 201 | 243 | 1 | 43 | LysM domain. The LysM (lysin motif) domain is about 40 residues long. It is found in a variety of enzymes involved in bacterial cell wall degradation. This domain may have a general peptidoglycan binding function. The structure of this domain is known. |
| pfam13472 | Lipase_GDSL_2 | 1.40e-12 | 301 | 454 | 31 | 176 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| cd00118 | LysM | 2.67e-11 | 199 | 242 | 1 | 45 | Lysin Motif is a small domain involved in binding peptidoglycan. LysM, a small globular domain with approximately 40 amino acids, is a widespread protein module involved in binding peptidoglycan in bacteria and chitin in eukaryotes. The domain was originally identified in enzymes that degrade bacterial cell walls, but proteins involved in many other biological functions also contain this domain. It has been reported that the LysM domain functions as a signal for specific plant-bacteria recognition in bacterial pathogenesis. Many of these enzymes are modular and are composed of catalytic units linked to one or several repeats of LysM domains. LysM domains are found in bacteria and eukaryotes. |
| COG1388 | LysM | 4.78e-11 | 160 | 248 | 30 | 116 | LysM repeat [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QIK56104.1 | 4.29e-139 | 1 | 475 | 1 | 490 |
| QIK61460.1 | 1.50e-138 | 22 | 475 | 3 | 475 |
| QSW87548.1 | 2.04e-127 | 17 | 474 | 25 | 473 |
| ABQ03283.1 | 1.49e-124 | 17 | 474 | 23 | 473 |
| AXG73103.1 | 1.98e-124 | 38 | 474 | 43 | 470 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4K3U_A | 2.96e-13 | 51 | 453 | 41 | 358 | PeptidoglycanO-acetylesterase in action, 30 min [Neisseria meningitidis 4119],4K3U_B Peptidoglycan O-acetylesterase in action, 30 min [Neisseria meningitidis 4119],4K40_A Peptidoglycan O-acetylesterase in action, 0 min [Neisseria meningitidis LNP21362],4K40_B Peptidoglycan O-acetylesterase in action, 0 min [Neisseria meningitidis LNP21362],4K7J_A Peptidoglycan O-acetylesterase in action, 5 min [Neisseria meningitidis 4119],4K7J_B Peptidoglycan O-acetylesterase in action, 5 min [Neisseria meningitidis 4119] |
| 4K9S_A | 3.97e-12 | 51 | 392 | 41 | 291 | PeptidoglycanO-acetylesterase in action, setmet [Neisseria meningitidis 4119],4K9S_B Peptidoglycan O-acetylesterase in action, setmet [Neisseria meningitidis 4119] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q49UX4 | 6.94e-07 | 193 | 294 | 21 | 126 | N-acetylmuramoyl-L-alanine amidase sle1 OS=Staphylococcus saprophyticus subsp. saprophyticus (strain ATCC 15305 / DSM 20229 / NCIMB 8711 / NCTC 7292 / S-41) OX=342451 GN=sle1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
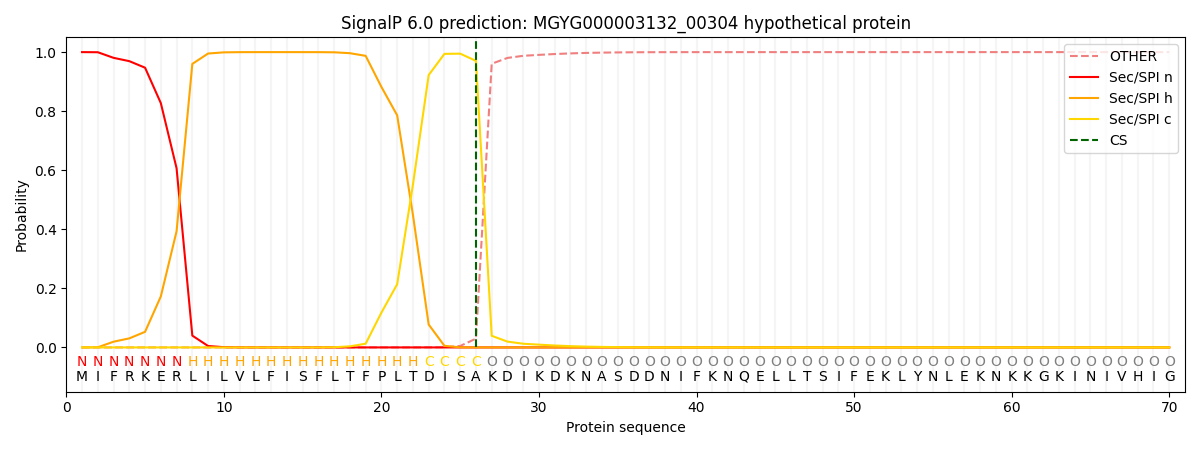
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000391 | 0.998841 | 0.000223 | 0.000184 | 0.000175 | 0.000155 |
