You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003164_00765
You are here: Home > Sequence: MGYG000003164_00765
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-485 sp002361235 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; CAG-485; CAG-485 sp002361235 | |||||||||||
| CAZyme ID | MGYG000003164_00765 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | Xylosidase/arabinosidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 33687; End: 35282 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 25 | 336 | 2e-102 | 0.9931972789115646 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18620 | GH43_XylA-like | 2.96e-102 | 35 | 345 | 1 | 270 | Glycosyl hydrolase family 43-like protein such as Clostridium stercorarium alpha-L-arabinofuranosidase XylA. This glycosyl hydrolase family 43 (GH43) subgroup belongs to the GH43_AXH-like subgroup which includes enzymes that have been characterized with beta-xylosidase (EC 3.2.1.37), alpha-L-arabinofuranosidase (EC 3.2.1.55), alpha-1,2-L-arabinofuranosidase 43A (arabinan-specific; EC 3.2.1.-), endo-alpha-L-arabinanase as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. The GH43_XylA-like subgroup includes Clostridium stercorarium alpha-L-arabinofuranosidase XylA, and enzymes that have been annotated as having beta-xylosidase (EC 3.2.1.37), alpha-L-arabinofuranosidase (EC 3.2.1.55), endo-alpha-L-arabinanase (EC 3.2.1.-) as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. |
| cd08990 | GH43_AXH_like | 2.12e-45 | 36 | 335 | 2 | 259 | Glycosyl hydrolase family 43 protein, includes arabinoxylan arabinofuranohydrolase, beta-xylosidase, endo-1,4-beta-xylanase, and alpha-L-arabinofuranosidase. This subgroup includes Bacillus subtilis arabinoxylan arabinofuranohydrolase (XynD;BsAXH-m23;BSU18160), Butyrivibrio proteoclasticus alpha-L-arabinofuranosidase (Xsa43E;bpr_I2319), Clostridium stercorarium alpha-L-arabinofuranosidase XylA, and metagenomic beta-xylosidase (EC 3.2.1.37) / alpha-L-arabinofuranosidase (EC 3.2.1.55) CoXyl43. It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. The GH43_AXH-like subgroup includes enzymes that have been characterized with beta-xylosidase, alpha-L-arabinofuranosidase, endo-alpha-L-arabinanase as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. Metagenomic beta-xylosidase/alpha-L-arabinofuranosidase CoXyl43 shows synergy with Trichoderma reesei cellulases and promotes plant biomass saccharification by degrading xylo-oligosaccharides, such as xylobiose and xylotriose, into the monosaccharide xylose. Studies show that the hydrolytic activity of CoXyl43 is stimulated in the presence of calcium. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd09003 | GH43_XynD-like | 2.99e-35 | 52 | 321 | 20 | 284 | Glycosyl hydrolase family 43 protein such as Bacillus subtilis arabinoxylan arabinofuranohydrolase (XynD;BsAXH-m23;BSU18160). This glycosyl hydrolase family 43 (GH43) subgroup includes characterized Bacillus subtilis arabinoxylan arabinofuranohydrolase (AXH), Caldicellulosiruptor sp. Tok7B.1 beta-1,4-xylanase (EC 3.2.1.8) / alpha-L-arabinosidase (EC 3.2.1.55) XynA, Caldicellulosiruptor sp. Rt69B.1 xylanase C (EC 3.2.1.8) XynC, and Caldicellulosiruptor saccharolyticus beta-xylosidase (EC 3.2.1.37)/ alpha-L-arabinofuranosidase (EC 3.2.1.55) XynF. It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. It belongs to the GH43_AXH-like subgroup which includes enzymes that have been annotated as having beta-xylosidase, alpha-L-arabinofuranosidase and arabinoxylan alpha-L-1,3-arabinofuranohydrolase, xylanase (endo-alpha-L-arabinanase) as well as AXH activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. Bacillus subtilis AXH (BsAXH-m2,3) has been shown to cleave arabinose units from O-2- or O-3-mono-substituted xylose residues and superposition of its structure with known structures of the GH43 exo-acting enzymes, beta-xylosidase and alpha-L-arabinanase, each in complex with their substrate, reveals a different orientation of the sugar backbone. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18619 | GH43_CoXyl43_like | 2.96e-28 | 52 | 318 | 19 | 284 | Glycosyl hydrolase family 43 protein such as metagenomic beta-xylosidase/alpha-L-arabinofuranosidase CoXyl43. This glycosyl hydrolase family 43 (GH43) subgroup belongs to the GH43_AXH-like subgroup which includes enzymes that have been characterized with beta-xylosidase (EC 3.2.1.37), alpha-L-arabinofuranosidase (EC 3.2.1.55), alpha-1,2-L-arabinofuranosidase 43A (arabinan-specific; EC 3.2.1.-), endo-alpha-L-arabinanase as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. Included in this subfamily is the metagenomic beta-xylosidase/alpha-L-arabinofuranosidase CoXyl43, which shows synergy with Trichoderma reesei cellulases and promotes plant biomass saccharification by degrading xylo-oligosaccharides, such as xylobiose and xylotriose, into the monosaccharide xylose. Studies show that the hydrolytic activity of CoXyl43 is stimulated in the presence of calcium. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18608 | GH43_F5-8_typeC-like | 1.22e-27 | 54 | 322 | 14 | 249 | Glycosyl hydrolase family 43 protein most having a F5/8 type C domain C-terminal to the GH43 domain. This glycosyl hydrolase family 43 (GH43) subgroup includes enzymes that have been annotated as having beta-xylosidase (EC 3.2.1.37), xylanase (EC 3.2.1.8), and beta-galactosidase (EC 3.2.1.145) activities, and some as F5/8 type C domain (also known as the discoidin (DS) domain)-containing proteins. Most contain a F5/8 type C domain C-terminal to the GH43 domain. It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. Characterized enzymes belonging to this subgroup include Lactobacillus brevis (LbAraf43) and Weissella sp (WAraf43) which show activity with similar catalytic efficiency on 1,5-alpha-L-arabinooligosaccharides with a degree of polymerization (DP) of 2-3; size is limited by an extended loop at the entrance to the active site. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADE83588.1 | 6.39e-205 | 25 | 529 | 42 | 550 |
| QCD35875.1 | 2.50e-204 | 15 | 531 | 21 | 541 |
| QVJ80144.1 | 2.58e-204 | 25 | 529 | 42 | 550 |
| AHF26057.1 | 6.00e-199 | 25 | 529 | 38 | 547 |
| BBK86497.1 | 6.46e-191 | 5 | 527 | 6 | 532 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5A8C_A | 9.89e-22 | 52 | 320 | 48 | 285 | ChainA, CARBOHYDRATE BINDING FAMILY 6 [Acetivibrio thermocellus],5A8D_A Chain A, CARBOHYDRATE BINDING FAMILY 6 [Acetivibrio thermocellus] |
| 4MLG_A | 1.72e-14 | 27 | 352 | 6 | 321 | Structureof RS223-Beta-xylosidase [uncultured organism],4MLG_B Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_C Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_D Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_E Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_F Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_G Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_H Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_I Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_J Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_K Structure of RS223-Beta-xylosidase [uncultured organism],4MLG_L Structure of RS223-Beta-xylosidase [uncultured organism] |
| 3C7E_A | 5.99e-14 | 52 | 518 | 33 | 474 | Crystalstructure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis. [Bacillus subtilis],3C7F_A Crystal structure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from bacillus subtilis in complex with xylotriose. [Bacillus subtilis],3C7H_A Crystal structure of glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis in complex with AXOS-4-0.5. [Bacillus subtilis],3C7O_A Crystal structure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis in complex with cellotetraose. [Bacillus subtilis] |
| 3C7G_A | 6.00e-14 | 52 | 518 | 34 | 475 | Crystalstructure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis in complex with xylotetraose. [Bacillus subtilis] |
| 6XN0_A | 5.95e-13 | 111 | 335 | 117 | 342 | ChainA, Xylosidase [Xanthomonas citri pv. citri str. 306],6XN0_B Chain B, Xylosidase [Xanthomonas citri pv. citri str. 306],6XN1_A Chain A, Xylosidase [Xanthomonas citri pv. citri str. 306],6XN1_B Chain B, Xylosidase [Xanthomonas citri pv. citri str. 306],6XN2_A Chain A, Xylosidase [Xanthomonas citri pv. citri str. 306],6XN2_B Chain B, Xylosidase [Xanthomonas citri pv. citri str. 306] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P48790 | 3.57e-45 | 25 | 527 | 7 | 468 | Xylosidase/arabinosidase OS=Thermoclostridium stercorarium OX=1510 GN=xylA PE=1 SV=1 |
| P45796 | 1.26e-16 | 36 | 529 | 56 | 507 | Arabinoxylan arabinofuranohydrolase OS=Paenibacillus polymyxa OX=1406 GN=xynD PE=1 SV=1 |
| Q45071 | 3.52e-13 | 52 | 518 | 59 | 500 | Arabinoxylan arabinofuranohydrolase OS=Bacillus subtilis (strain 168) OX=224308 GN=xynD PE=1 SV=2 |
| P48791 | 1.26e-12 | 142 | 338 | 112 | 310 | Beta-xylosidase OS=Prevotella ruminicola OX=839 GN=xynB PE=3 SV=1 |
| P49943 | 1.34e-12 | 27 | 318 | 7 | 290 | Xylosidase/arabinosidase OS=Bacteroides ovatus OX=28116 GN=xsa PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
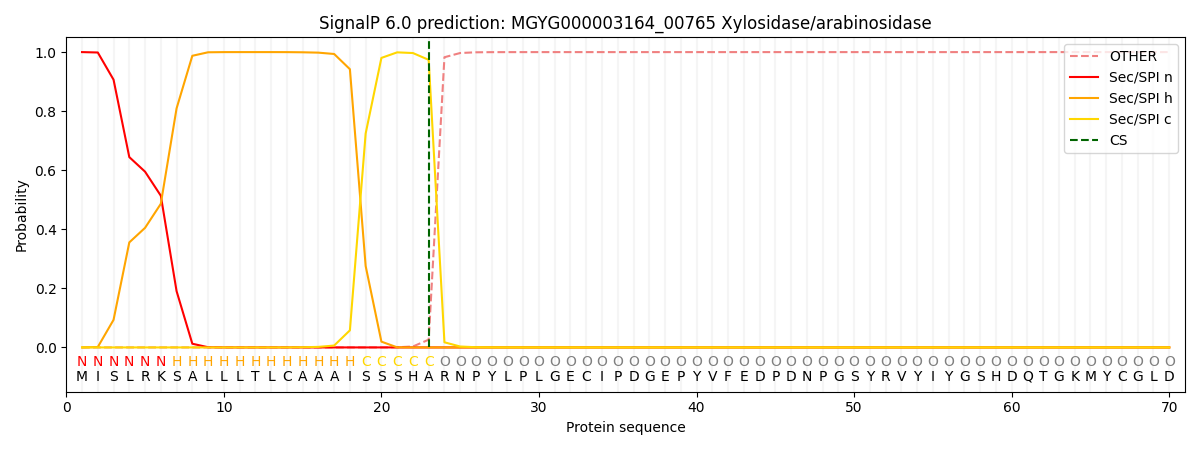
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000235 | 0.999148 | 0.000159 | 0.000169 | 0.000147 | 0.000132 |
