You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003252_00403
You are here: Home > Sequence: MGYG000003252_00403
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides sp900761785 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp900761785 | |||||||||||
| CAZyme ID | MGYG000003252_00403 | |||||||||||
| CAZy Family | GH51 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 67659; End: 70067 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH51 | 362 | 800 | 4.8e-81 | 0.6936507936507936 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3534 | AbfA | 2.64e-47 | 376 | 796 | 46 | 501 | Alpha-L-arabinofuranosidase [Carbohydrate transport and metabolism]. |
| smart00813 | Alpha-L-AF_C | 1.26e-12 | 618 | 787 | 1 | 189 | Alpha-L-arabinofuranosidase C-terminus. This entry represents the C terminus (approximately 200 residues) of bacterial and eukaryotic alpha-L-arabinofuranosidase. This catalyses the hydrolysis of non-reducing terminal alpha-L-arabinofuranosidic linkages in L-arabinose-containing polysaccharides. |
| pfam06964 | Alpha-L-AF_C | 4.94e-12 | 627 | 787 | 24 | 192 | Alpha-L-arabinofuranosidase C-terminal domain. This family represents the C-terminus (approximately 200 residues) of bacterial and eukaryotic alpha-L-arabinofuranosidase (EC:3.2.1.55). This catalyzes the hydrolysis of nonreducing terminal alpha-L-arabinofuranosidic linkages in L-arabinose-containing polysaccharides. |
| pfam06439 | DUF1080 | 6.37e-08 | 104 | 234 | 51 | 182 | Domain of Unknown Function (DUF1080). This family has structural similarity to an endo-1,3-1,4-beta glucanase belonging to glycoside hydrolase family 16. However, the structure surrounding the active site differs from that of the endo-1,3-1,4-beta glucanase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AII66310.1 | 0.0 | 10 | 796 | 9 | 794 |
| QJR54187.1 | 0.0 | 10 | 796 | 9 | 794 |
| QUT85430.1 | 0.0 | 10 | 796 | 9 | 794 |
| QJR61015.1 | 0.0 | 10 | 796 | 9 | 794 |
| AND18135.1 | 0.0 | 10 | 796 | 9 | 794 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4ATW_A | 1.78e-44 | 376 | 794 | 45 | 482 | Thecrystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_B The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_C The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_D The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_E The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_F The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8] |
| 3S2C_A | 2.51e-44 | 376 | 794 | 45 | 482 | Structureof the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_B Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_C Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_D Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_E Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_F Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_G Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_H Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_I Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_J Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_K Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_L Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1] |
| 3UG3_A | 2.64e-44 | 376 | 794 | 65 | 502 | Crystalstructure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG4_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG5_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima] |
| 5O7Z_A | 4.28e-25 | 373 | 622 | 42 | 294 | ChainA, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_B Chain B, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_C Chain C, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_D Chain D, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_E Chain E, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O7Z_F Chain F, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_A Chain A, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_B Chain B, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_C Chain C, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_D Chain D, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_E Chain E, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila],5O80_F Chain F, Intracellular exo-alpha-(1->5)-L-arabinofuranosidase [Thermochaetoides thermophila] |
| 2C7F_A | 4.73e-25 | 373 | 622 | 53 | 305 | ChainA, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C7F_B Chain B, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C7F_C Chain C, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C7F_D Chain D, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C7F_E Chain E, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C7F_F Chain F, Alpha-l-arabinofuranosidase [Acetivibrio thermocellus],2C8N_A Chain A, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus],2C8N_B Chain B, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus],2C8N_C Chain C, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus],2C8N_D Chain D, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus],2C8N_E Chain E, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus],2C8N_F Chain F, ALPHA-L-ARABINOFURANOSIDASE [Acetivibrio thermocellus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9SG80 | 2.73e-35 | 284 | 789 | 153 | 651 | Alpha-L-arabinofuranosidase 1 OS=Arabidopsis thaliana OX=3702 GN=ASD1 PE=1 SV=1 |
| Q8VZR2 | 6.22e-32 | 284 | 789 | 152 | 648 | Alpha-L-arabinofuranosidase 2 OS=Arabidopsis thaliana OX=3702 GN=ASD2 PE=2 SV=1 |
| A1CQC3 | 8.97e-30 | 376 | 769 | 55 | 481 | Probable alpha-L-arabinofuranosidase C OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=abfC PE=3 SV=2 |
| B0XQB2 | 9.32e-29 | 376 | 549 | 55 | 221 | Probable alpha-L-arabinofuranosidase C OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=abfC PE=3 SV=2 |
| Q4WTB3 | 9.32e-29 | 376 | 549 | 55 | 221 | Probable alpha-L-arabinofuranosidase C OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=abfC PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
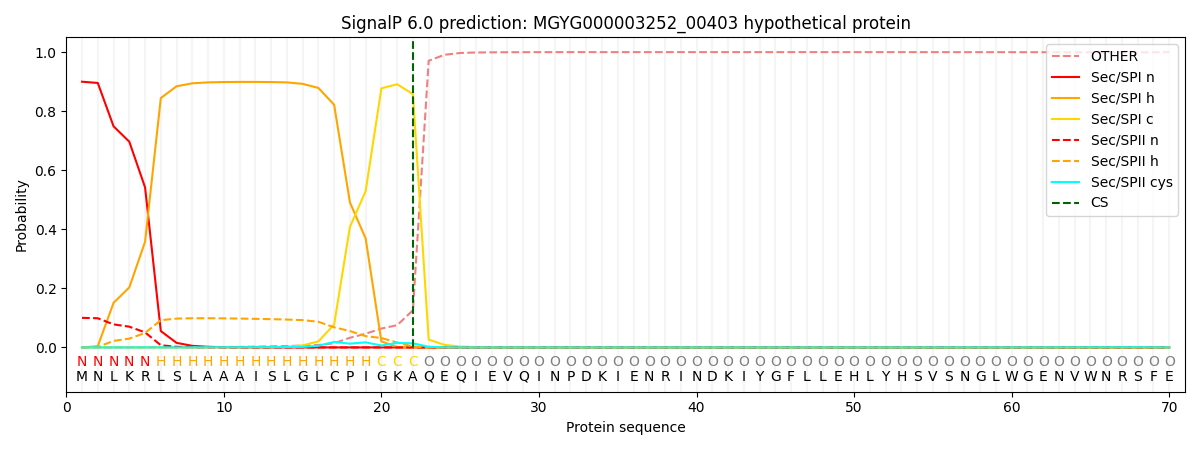
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000741 | 0.893414 | 0.105061 | 0.000269 | 0.000239 | 0.000230 |
