You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003273_00173
You are here: Home > Sequence: MGYG000003273_00173
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; RUG11200; | |||||||||||
| CAZyme ID | MGYG000003273_00173 | |||||||||||
| CAZy Family | GH13 | |||||||||||
| CAZyme Description | 1,4-alpha-glucan branching enzyme GlgB | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 201; End: 2657 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 66 | 321 | 8.2e-72 | 0.9924812030075187 |
| CBM26 | 569 | 632 | 1.8e-16 | 0.84 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd11315 | AmyAc_bac1_AmyA | 3.94e-106 | 57 | 395 | 1 | 352 | Alpha amylase catalytic domain found in bacterial Alpha-amylases (also called 1,4-alpha-D-glucan-4-glucanohydrolase). AmyA (EC 3.2.1.1) catalyzes the hydrolysis of alpha-(1,4) glycosidic linkages of glycogen, starch, related polysaccharides, and some oligosaccharides. This group includes Firmicutes, Proteobacteria, Actinobacteria, and Cyanobacteria. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| NF033930 | pneumo_PspA | 3.37e-53 | 632 | 813 | 476 | 660 | pneumococcal surface protein A. The pneumococcal surface protein proteins, found in Streptococcus pneumoniae, are repetitive, with patterns of localized high sequence identity across pairs of proteins given different specific names that recombination may be presumed. This protein, PspA, has an N-terminal region that lacks a cross-wall-targeting YSIRK type extended signal peptide, in contrast to the closely related choline-binding protein CbpA which has a similar C-terminus but a YSIRK-containing region at the N-terminus. |
| NF033838 | PspC_subgroup_1 | 9.42e-52 | 640 | 813 | 502 | 683 | pneumococcal surface protein PspC, choline-binding form. The pneumococcal surface protein PspC, as described in Streptococcus pneumoniae, is a repetitive and highly variable protein, recognized by a conserved N-terminal domain and also by genomic location. This form, subgroup 1, has variable numbers of a choline-binding repeat in the C-terminal region, and is also known as choline-binding protein A. The other form, subgroup 2, is anchored covalently after cleavage by sortase at a C-terminal LPXTG site. |
| NF033930 | pneumo_PspA | 5.28e-48 | 656 | 810 | 442 | 599 | pneumococcal surface protein A. The pneumococcal surface protein proteins, found in Streptococcus pneumoniae, are repetitive, with patterns of localized high sequence identity across pairs of proteins given different specific names that recombination may be presumed. This protein, PspA, has an N-terminal region that lacks a cross-wall-targeting YSIRK type extended signal peptide, in contrast to the closely related choline-binding protein CbpA which has a similar C-terminus but a YSIRK-containing region at the N-terminus. |
| NF033838 | PspC_subgroup_1 | 1.38e-46 | 656 | 810 | 485 | 642 | pneumococcal surface protein PspC, choline-binding form. The pneumococcal surface protein PspC, as described in Streptococcus pneumoniae, is a repetitive and highly variable protein, recognized by a conserved N-terminal domain and also by genomic location. This form, subgroup 1, has variable numbers of a choline-binding repeat in the C-terminal region, and is also known as choline-binding protein A. The other form, subgroup 2, is anchored covalently after cleavage by sortase at a C-terminal LPXTG site. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QFJ55411.1 | 4.07e-139 | 46 | 470 | 43 | 470 |
| QNT67433.1 | 2.06e-126 | 38 | 556 | 17 | 529 |
| QUB83913.1 | 9.11e-117 | 46 | 487 | 22 | 450 |
| AET60999.1 | 1.26e-106 | 5 | 657 | 1 | 659 |
| QDY86356.1 | 3.34e-106 | 5 | 656 | 1 | 658 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3DC0_A | 5.91e-70 | 56 | 467 | 4 | 422 | Crystalstructure of native alpha-amylase from Bacillus sp. KR-8104 [Bacillus sp. KR-8104] |
| 1UA7_A | 5.87e-69 | 56 | 467 | 4 | 422 | ChainA, Alpha-amylase [Bacillus subtilis] |
| 1BAG_A | 8.83e-69 | 52 | 467 | 3 | 425 | ChainA, ALPHA-1,4-GLUCAN-4-GLUCANOHYDROLASE [Bacillus subtilis] |
| 1XGZ_A | 2.30e-19 | 54 | 464 | 5 | 477 | ChainA, Alpha-amylase, pancreatic [Homo sapiens],1XH0_A Chain A, Alpha-amylase, pancreatic [Homo sapiens],1XH1_A Chain A, Alpha-amylase, pancreatic [Homo sapiens],1XH2_A Chain A, Alpha-amylase, pancreatic [Homo sapiens],3BAK_A Chain A, Pancreatic alpha-amylase [Homo sapiens],3BAX_A Chain A, Pancreatic alpha-amylase [Homo sapiens],3BAY_A Chain A, Pancreatic alpha-amylase [Homo sapiens] |
| 1BSI_A | 5.39e-19 | 54 | 464 | 5 | 477 | HUMANPANCREATIC ALPHA-AMYLASE FROM PICHIA PASTORIS, GLYCOSYLATED PROTEIN [Homo sapiens],1CPU_A SUBSITE MAPPING OF THE ACTIVE SITE OF HUMAN PANCREATIC ALPHA-AMYLASE USING SUBSTRATES, THE PHARMACOLOGICAL INHIBITOR ACARBOSE, AND AN ACTIVE SITE VARIANT [Homo sapiens],1HNY_A The structure of human pancreatic alpha-amylase at 1.8 angstroms resolution and comparisons with related enzymes [Homo sapiens],1U2Y_A In situ extension as an approach for identifying novel alpha-amylase inhibitors, structure containing D-gluconhydroximo-1,5-lactam [Homo sapiens],1U30_A In situ extension as an approach for identifying novel alpha-amylase inhibitors, structure containing maltosyl-alpha (1,4)-D-gluconhydroximo-1,5-lactam [Homo sapiens],1U33_A In situ extension as an approach for identifying novel alpha-amylase inhibitors [Homo sapiens],1XCW_A Acarbose Rearrangement Mechanism Implied by the Kinetic and Structural Analysis of Human Pancreatic alpha-Amylase in Complex with Analogues and Their Elongated Counterparts [Homo sapiens],1XCX_A Acarbose Rearrangement Mechanism Implied by the Kinetic and Structural Analysis of Human Pancreatic alpha-Amylase in Complex with Analogues and Their Elongated Counterparts [Homo sapiens],1XD0_A Acarbose Rearrangement Mechanism Implied by the Kinetic and Structural Analysis of Human Pancreatic alpha-Amylase in Complex with Analogues and Their Elongated Counterparts [Homo sapiens],1XD1_A Acarbose Rearrangement Mechanism Implied by the Kinetic and Structural Analysis of Human Pancreatic alpha-Amylase in Complex with Analogues and Their Elongated Counterparts [Homo sapiens],2QMK_A Human pancreatic alpha-amylase complexed with nitrite [Homo sapiens],2QV4_A Human pancreatic alpha-amylase complexed with nitrite and acarbose [Homo sapiens],3BAI_A Human Pancreatic Alpha Amylase with Bound Nitrate [Homo sapiens],3BAJ_A Human Pancreatic Alpha-Amylase in Complex with Nitrate and Acarbose [Homo sapiens],3BAW_A Human pancreatic alpha-amylase complexed with azide [Homo sapiens],3IJ7_A Directed 'in situ' Elongation as a Strategy to Characterize the Covalent Glycosyl-Enzyme Catalytic Intermediate of Human Pancreatic a-Amylase [Homo sapiens],3IJ8_A Directed 'in situ' Elongation as a Strategy to Characterize the Covalent Glycosyl-Enzyme Catalytic Intermediate of Human Pancreatic a-Amylase [Homo sapiens],3IJ9_A Directed 'in situ' Elongation as a Strategy to Characterize the Covalent Glycosyl-Enzyme Catalytic Intermediate of Human Pancreatic a-Amylase [Homo sapiens],4GQQ_A Human pancreatic alpha-amylase with bound ethyl caffeate [Homo sapiens],4GQR_A Human Pancreatic alpha-amylase in complex with myricetin [Homo sapiens],4W93_A Human pancreatic alpha-amylase in complex with montbretin A [Homo sapiens],4X9Y_A Wild-Type Human Pancreatic Alpha-Amylase at True Atomic Resolution (1.07 A) [Homo sapiens],5E0F_A Human pancreatic alpha-amylase in complex with mini-montbretin A [Homo sapiens],5EMY_A Human Pancreatic Alpha-Amylase in complex with the mechanism based inactivator glucosyl epi-cyclophellitol [Homo sapiens],5KEZ_A Selective and potent inhibition of the glycosidase human amylase by the short and extremely compact peptide piHA from mRNA display [Homo sapiens],5U3A_A Ultra High Resolution Crystal Structure of Human Pancreatic Alpha Amylase [Homo sapiens],5VA9_A Human pancreatic alpha amylase in complex with peptide inhibitor piHA-L5(d10Y) [Homo sapiens],5VA9_B Human pancreatic alpha amylase in complex with peptide inhibitor piHA-L5(d10Y) [Homo sapiens],6OBX_A Montbretin A analogue M10-MbA in complex with Human pancreatic alpha-amylase [Homo sapiens],6OCN_A Montbretin A analogue M06-MbA in complex with Human pancreatic alpha-amylase [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P00691 | 4.44e-84 | 40 | 635 | 34 | 639 | Alpha-amylase OS=Bacillus subtilis (strain 168) OX=224308 GN=amyE PE=1 SV=2 |
| P23671 | 3.76e-54 | 45 | 467 | 41 | 463 | Alpha-amylase OS=Clostridium acetobutylicum (strain ATCC 824 / DSM 792 / JCM 1419 / LMG 5710 / VKM B-1787) OX=272562 GN=amyA PE=3 SV=2 |
| P30269 | 1.53e-46 | 42 | 644 | 132 | 787 | Alpha-amylase OS=Butyrivibrio fibrisolvens OX=831 GN=amyA PE=3 SV=1 |
| P22630 | 1.83e-20 | 60 | 406 | 24 | 400 | Alpha-amylase OS=Aeromonas hydrophila OX=644 PE=3 SV=1 |
| P19961 | 1.37e-18 | 54 | 464 | 20 | 492 | Alpha-amylase 2B OS=Homo sapiens OX=9606 GN=AMY2B PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
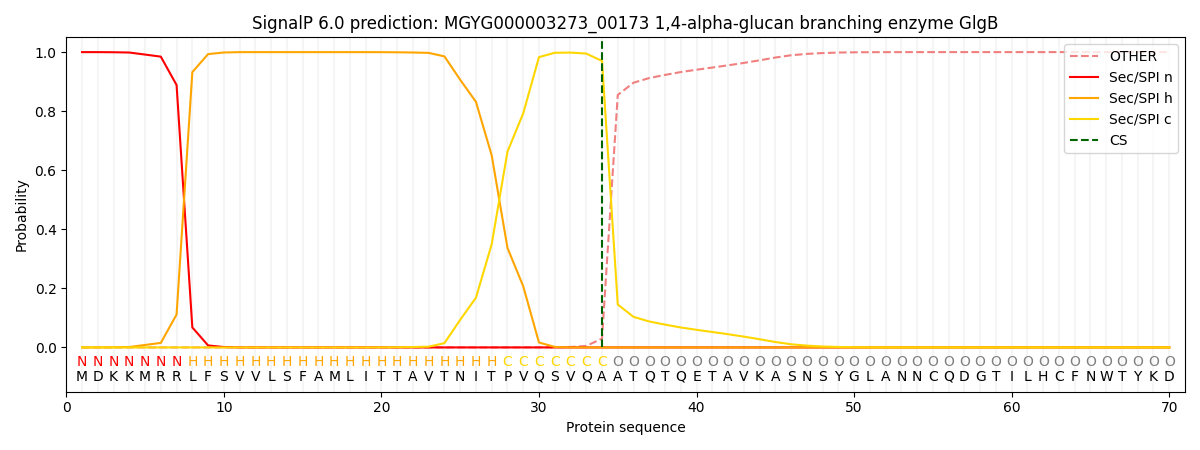
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000328 | 0.998954 | 0.000163 | 0.000204 | 0.000161 | 0.000147 |

