You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003337_00622
You are here: Home > Sequence: MGYG000003337_00622
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Ruminococcus_C sp900765125 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus_C; Ruminococcus_C sp900765125 | |||||||||||
| CAZyme ID | MGYG000003337_00622 | |||||||||||
| CAZy Family | CBM4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 3329; End: 5671 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH9 | 286 | 713 | 5.8e-114 | 0.9952153110047847 |
| CBM4 | 33 | 158 | 1.1e-31 | 0.9920634920634921 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00759 | Glyco_hydro_9 | 2.32e-96 | 289 | 712 | 2 | 374 | Glycosyl hydrolase family 9. |
| cd02850 | E_set_Cellulase_N | 1.28e-23 | 197 | 281 | 2 | 86 | N-terminal Early set domain associated with the catalytic domain of cellulase. E or "early" set domains are associated with the catalytic domain of cellulases at the N-terminal end. Cellulases are O-glycosyl hydrolases (GHs) that hydrolyze beta 1-4 glucosidic bonds in cellulose. They are usually categorized into either exoglucanases, which sequentially release terminal sugar units from the cellulose chain, or endoglucanases, which also attack the chain internally. The N-terminal domain of cellulase may be related to the immunoglobulin and/or fibronectin type III superfamilies. These domains are associated with different types of catalytic domains at either the N-terminal or C-terminal end and may be involved in homodimeric/tetrameric/dodecameric interactions. Members of this family include members of the alpha amylase family, sialidase, galactose oxidase, cellulase, cellulose, hyaluronate lyase, chitobiase, and chitinase, among others. |
| pfam02927 | CelD_N | 1.77e-23 | 195 | 272 | 1 | 80 | Cellulase N-terminal ig-like domain. |
| PLN00119 | PLN00119 | 1.53e-15 | 326 | 649 | 68 | 413 | endoglucanase |
| cd14256 | Dockerin_I | 6.82e-14 | 723 | 779 | 1 | 57 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBL16782.1 | 0.0 | 1 | 771 | 1 | 779 |
| AGF55906.1 | 6.47e-237 | 33 | 713 | 38 | 722 |
| AQR94654.1 | 1.30e-236 | 33 | 713 | 38 | 722 |
| ADZ82408.1 | 2.78e-225 | 29 | 708 | 32 | 717 |
| QEH68101.1 | 4.06e-225 | 29 | 708 | 32 | 717 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4CJ0_A | 5.48e-105 | 193 | 759 | 26 | 595 | ChainA, ENDOGLUCANASE D [Acetivibrio thermocellus],4CJ1_A Chain A, ENDOGLUCANASE D [Acetivibrio thermocellus] |
| 1CLC_A | 7.97e-105 | 193 | 759 | 40 | 609 | ChainA, ENDOGLUCANASE CELD; EC: 3.2.1.4 [Acetivibrio thermocellus] |
| 5E2J_A | 5.91e-95 | 253 | 713 | 80 | 552 | Crystalstructure of single mutant thermostable endoglucanase (D468A) from Alicyclobacillus acidocaldarius [Alicyclobacillus acidocaldarius subsp. acidocaldarius],5E2J_B Crystal structure of single mutant thermostable endoglucanase (D468A) from Alicyclobacillus acidocaldarius [Alicyclobacillus acidocaldarius subsp. acidocaldarius] |
| 3EZ8_A | 9.22e-95 | 253 | 713 | 57 | 529 | CrystalStructure of endoglucanase Cel9A from the thermoacidophilic Alicyclobacillus acidocaldarius [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3GZK_A Structure of A. Acidocaldarius Cellulase CelA [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3H2W_A Structure of A. acidocaldarius cellulase CelA in complex with cellobiose [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3H3K_A Structure of A. acidocaldarius cellulase CelA in complex with cellotetraose [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3RX5_A structure of AaCel9A in complex with cellotriose-like isofagomine [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3RX7_A Structure of AaCel9A in complex with cellotetraose-like isofagomine [Alicyclobacillus acidocaldarius subsp. acidocaldarius],3RX8_A structure of AaCel9A in complex with cellobiose-like isofagomine [Alicyclobacillus acidocaldarius subsp. acidocaldarius] |
| 3X17_A | 1.08e-89 | 197 | 713 | 18 | 554 | Crystalstructure of metagenome-derived glycoside hydrolase family 9 endoglucanase [uncultured bacterium],3X17_B Crystal structure of metagenome-derived glycoside hydrolase family 9 endoglucanase [uncultured bacterium] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P0C2S4 | 3.00e-104 | 193 | 759 | 26 | 595 | Endoglucanase D (Fragment) OS=Acetivibrio thermocellus OX=1515 GN=celD PE=1 SV=1 |
| A3DDN1 | 7.96e-104 | 193 | 759 | 50 | 619 | Endoglucanase D OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=celD PE=1 SV=1 |
| P23658 | 2.88e-97 | 197 | 713 | 4 | 543 | Cellodextrinase OS=Butyrivibrio fibrisolvens OX=831 GN=ced1 PE=1 SV=1 |
| P0C2S1 | 3.33e-87 | 47 | 745 | 56 | 854 | Cellulose 1,4-beta-cellobiosidase OS=Acetivibrio thermocellus OX=1515 GN=celK PE=1 SV=1 |
| A3DCH1 | 8.85e-87 | 47 | 745 | 56 | 854 | Cellulose 1,4-beta-cellobiosidase OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=celK PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
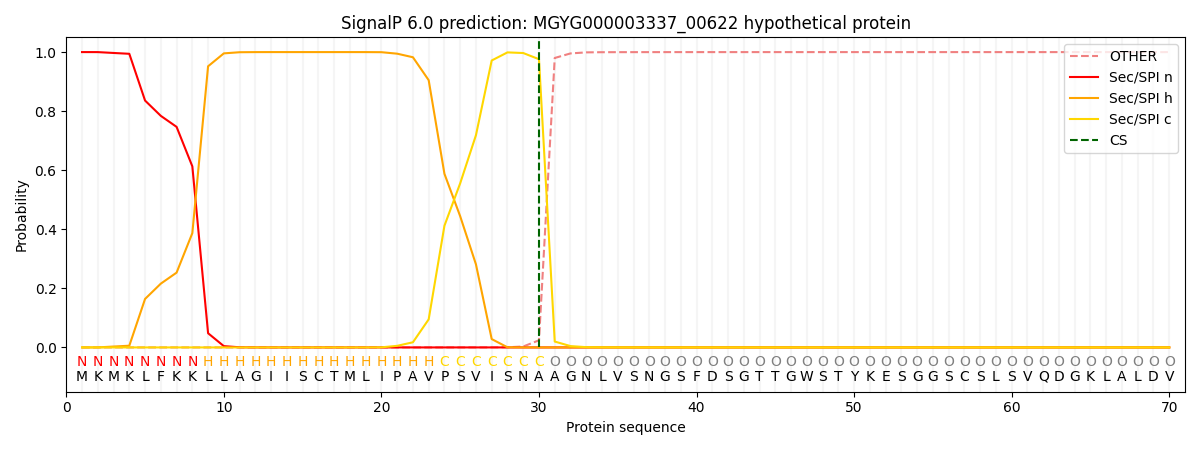
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000230 | 0.999133 | 0.000179 | 0.000185 | 0.000152 | 0.000133 |
