You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003355_04003
You are here: Home > Sequence: MGYG000003355_04003
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Lachnoclostridium phytofermentans_A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Lachnoclostridium; Lachnoclostridium phytofermentans_A | |||||||||||
| CAZyme ID | MGYG000003355_04003 | |||||||||||
| CAZy Family | GH33 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1559; End: 2572 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 27 | 332 | 1e-29 | 0.9035087719298246 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam13088 | BNR_2 | 5.35e-74 | 30 | 321 | 1 | 280 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
| COG4692 | COG4692 | 3.16e-44 | 19 | 332 | 33 | 368 | Predicted neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd15482 | Sialidase_non-viral | 2.48e-42 | 7 | 336 | 1 | 339 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| cd18621 | GH32_XdINV-like | 0.008 | 244 | 295 | 72 | 125 | glycoside hydrolase family 32 protein such as Xanthophyllomyces dendrorhous beta-fructofuranosidase (Inv;Xd-INV;XdINV). This subfamily of glycosyl hydrolase family GH32 includes fructan:fructan 1-fructosyltransferase (FT, EC 2.4.1.100) and beta-fructofuranosidase (invertase or Inv, EC 3.2.1.26), among others. These enzymes cleave sucrose into fructose and glucose via beta-fructofuranosidase activity, producing invert sugar that is a mixture of dextrorotatory D-glucose and levorotatory D-fructose, thus named invertase (EC 3.2.1.26). These retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. Xanthophyllomyces dendrorhous beta-fructofuranosidase (XdINV) also catalyzes the synthesis of fructooligosaccharides (FOS, a beneficial prebiotic), producing neo-FOS, making it an interesting biotechnology target. Structural studies show plasticity of its active site, having a flexible loop that is essential in binding sucrose and beta(2-1)-linked oligosaccharide, making it a valuable biocatalyst to produce novel bioconjugates. The breakdown of sucrose is widely used as a carbon or energy source by bacteria, fungi, and plants. Invertase is used commercially in the confectionery industry, since fructose has a sweeter taste than sucrose and a lower tendency to crystallize. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AUS95056.1 | 5.80e-104 | 2 | 333 | 3 | 329 |
| ALS21221.1 | 2.97e-97 | 3 | 332 | 4 | 332 |
| AEL25808.1 | 6.02e-96 | 1 | 333 | 45 | 361 |
| AKP51429.1 | 8.00e-96 | 1 | 333 | 43 | 359 |
| AZS14954.1 | 2.20e-94 | 4 | 332 | 5 | 331 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4YW1_A | 1.47e-07 | 83 | 242 | 400 | 540 | ChainA, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW1_B Chain B, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW2_A Chain A, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW2_B Chain B, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW3_A Chain A, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW3_B Chain B, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW4_A Streptococcus pneumoniae sialidase NanC [Streptococcus pneumoniae],4YW4_B Streptococcus pneumoniae sialidase NanC [Streptococcus pneumoniae],4YW5_A Chain A, Neuraminidase C [Streptococcus pneumoniae TIGR4],4YW5_B Chain B, Neuraminidase C [Streptococcus pneumoniae TIGR4],5F9T_A Chain A, Neuraminidase C [Streptococcus pneumoniae TIGR4],5F9T_B Chain B, Neuraminidase C [Streptococcus pneumoniae TIGR4] |
| 4YZ1_A | 1.48e-07 | 83 | 242 | 419 | 559 | CrystalStructure of Streptococcus pneumoniae NanC, apo structure. [Streptococcus pneumoniae TIGR4],4YZ1_B Crystal Structure of Streptococcus pneumoniae NanC, apo structure. [Streptococcus pneumoniae TIGR4],4YZ2_A Crystal Structure of Streptococcus pneumoniae NanC, in complex with 2-deoxy-2,3-didehydro-N-acetylneuraminic acid. [Streptococcus pneumoniae],4YZ2_B Crystal Structure of Streptococcus pneumoniae NanC, in complex with 2-deoxy-2,3-didehydro-N-acetylneuraminic acid. [Streptococcus pneumoniae],4YZ3_A Crystal Structure of Streptococcus pneumoniae NanC, in complex with Oseltamivir. [Streptococcus pneumoniae TIGR4],4YZ3_B Crystal Structure of Streptococcus pneumoniae NanC, in complex with Oseltamivir. [Streptococcus pneumoniae TIGR4],4YZ4_A Crystal Structure of Streptococcus pneumoniae NanC, in complex with N-Acetylneuraminic acid. [Streptococcus pneumoniae],4YZ4_B Crystal Structure of Streptococcus pneumoniae NanC, in complex with N-Acetylneuraminic acid. [Streptococcus pneumoniae],4YZ5_A Crystal Structure of Streptococcus pneumoniae NanC, in complex with 3-Sialyllactose [Streptococcus pneumoniae],4YZ5_B Crystal Structure of Streptococcus pneumoniae NanC, in complex with 3-Sialyllactose [Streptococcus pneumoniae] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as OTHER
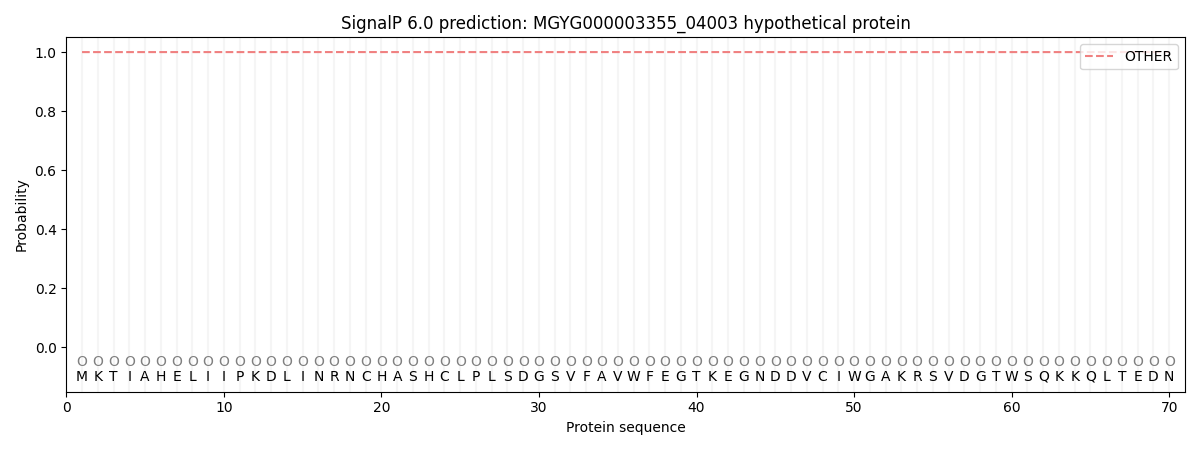
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000043 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
