You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003359_05122
You are here: Home > Sequence: MGYG000003359_05122
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
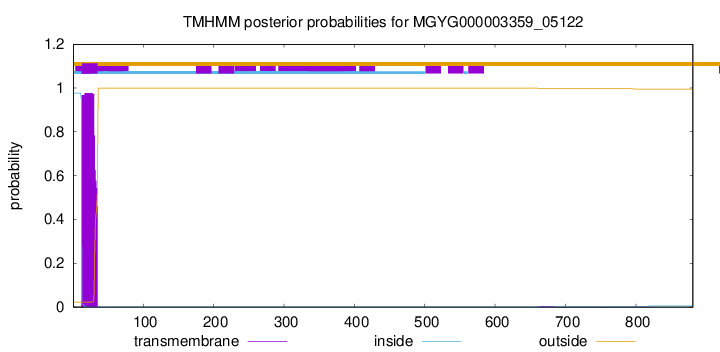
TMHMM annotations
Basic Information help
| Species | Paenibacillus_A sp900766135 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Paenibacillales; Paenibacillaceae; Paenibacillus_A; Paenibacillus_A sp900766135 | |||||||||||
| CAZyme ID | MGYG000003359_05122 | |||||||||||
| CAZy Family | CBM35 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 52835; End: 55480 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM35 | 63 | 173 | 6.5e-20 | 0.9327731092436975 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd04081 | CBM35_galactosidase-like | 7.94e-29 | 52 | 173 | 1 | 125 | Carbohydrate Binding Module family 35 (CBM35); appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. This family includes carbohydrate binding module family 35 (CBM35); these are non-catalytic carbohydrate binding domains that are appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. Examples of proteins which contain CBM35s belonging to this family includes the CBM35 of an exo-beta-1,3-galactanase from Phanerochaete chrysosporium 9 (Pc1,3Gal43A) which is appended to a GH43 domain, and the CBM35 domain of two bifunctional proteins with beta-L-arabinopyranosidase/alpha-D-galactopyranosidase activities from Fusarium oxysporum 12S, Foap1 and Foap2 (Fo/AP1 and Fo/AP2), that are appended to GH27 domains. CBM35s are unique in that they display conserved specificity through extensive sequence similarity but divergent function through their appended catalytic modules. They are known to bind alpha-D-galactose (Gal), mannan (Man), xylan, glucuronic acid (GlcA), a beta-polymer of mannose, and possibly glucans, forming four subfamilies based on general ligand specificities (galacto, urono, manno, and gluco configurations). Some CBM35s bind their ligands in a calcium-dependent manner. In contrast to most CBMs that are generally rigid proteins, CBM35 undergoes significant conformational change upon ligand binding. GH43 includes beta-xylosidases and beta-xylanases, using aryl-glycosides as substrates, while family GH27 includes alpha-galactosidases, alpha-N-acetylgalactosaminidases, and isomaltodextranases. |
| cd14791 | GH36 | 1.98e-18 | 487 | 768 | 3 | 286 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd14792 | GH27 | 2.84e-15 | 487 | 782 | 2 | 268 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd04083 | CBM35_Lmo2446-like | 1.18e-09 | 76 | 173 | 24 | 125 | Carbohydrate Binding Module 35 (CBM35) domains similar to Lmo2446. This family includes carbohydrate binding module 35 (CBM35) domains that are appended to several carbohydrate binding enzymes. Some CBM35 domains belonging to this family are appended to glycoside hydrolase (GH) family domains, including glycoside hydrolase family 31 (GH31), for example the CBM35 domain of Lmo2446, an uncharacterized protein from Listeria monocytogenes EGD-e. These CBM35s are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH catalytic modules with their dedicated, insoluble substrates. GH31 has a wide range of hydrolytic activities such as alpha-glucosidase, alpha-xylosidase, 6-alpha-glucosyltransferase, or alpha-1,4-glucan lyase, cleaving a terminal carbohydrate moiety from a substrate that may be a starch or a glycoprotein. Most characterized GH31 enzymes are alpha-glucosidases. |
| cd04080 | CBM6_cellulase-like | 2.41e-06 | 71 | 174 | 40 | 144 | Carbohydrate Binding Module 6 (CBM6); appended to glycoside hydrolase (GH) domains, including GH5 (cellulase). This family includes carbohydrate binding module 6 (CBM6) domains that are appended to several glycoside hydrolase (GH) domains, including GH5 (cellulase) and GH16, as well as to coagulation factor 5/8 carbohydrate-binding domains. CBM6s are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH catalytic modules with their dedicated, insoluble substrates. The CBM6s are appended to GHs that display a diversity of substrate specificities. For some members of this family information is available about the specific substrates of the appended GH domains. It includes the CBM domains of various enzymes involved in cell wall degradation including, an extracellular beta-1,3-glucanase from Lysobacter enzymogenes encoded by the gluC gene (its catalytic domain belongs to the GH16 family), the tandem CBM domains of Pseudomonas sp. PE2 beta-1,3(4)-glucanase A (its catalytic domain also belongs to GH16), and a family 6 CBM from Cellvibrio mixtus Endoglucanase 5A (CmCBM6) which binds to the beta1,4-beta1,3-mixed linked glucans lichenan, and barley beta-glucan, cello-oligosaccharides, insoluble forms of cellulose, the beta1,3-glucan laminarin, and xylooligosaccharides, and the CBM6 of Fibrobacter succinogenes S85 XynD xylanase, appended to a GH10 domain, and Cellvibrio japonicas Cel5G appended to a GH5 (cellulase) domain. GH5 (cellulase) family includes enzymes with several known activities such as endoglucanase, beta-mannanase, and xylanase, which are involved in the degradation of cellulose and xylans. GH16 family includes enzymes with lichenase, xyloglucan endotransglycosylase (XET), and beta-agarase activities. CBM6 is an unusual CBM as it represents a chimera of two distinct binding sites with different modes of binding: binding site I within the loop regions and binding site II on the concave face of the beta-sandwich fold. For CmCBM6 it has been shown that these two binding sites have different ligand specificities. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANS74346.1 | 0.0 | 22 | 877 | 21 | 868 |
| AYB43259.1 | 0.0 | 46 | 876 | 40 | 867 |
| AWP26688.1 | 0.0 | 49 | 876 | 44 | 868 |
| QOT11184.1 | 0.0 | 46 | 876 | 40 | 867 |
| ACX66630.1 | 0.0 | 46 | 876 | 40 | 867 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2WZ8_A | 8.73e-13 | 45 | 176 | 2 | 149 | ChainA, Cellulosome Protein Dockerin Type I [Acetivibrio thermocellus] |
| 5AWO_A | 4.69e-08 | 549 | 860 | 113 | 455 | Arthrobacterglobiformis T6 isomalto-dextranse [Arthrobacter globiformis],5AWP_A Arthrobacter globiformis T6 isomalto-dextranase complexed with isomaltose [Arthrobacter globiformis],5AWQ_A Arthrobacter globiformis T6 isomalto-dextranse complexed with panose [Arthrobacter globiformis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q44052 | 1.53e-07 | 549 | 860 | 139 | 481 | Isomalto-dextranase OS=Arthrobacter globiformis OX=1665 GN=imd PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002535 | 0.663594 | 0.332349 | 0.000873 | 0.000391 | 0.000223 |