You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003362_01852
You are here: Home > Sequence: MGYG000003362_01852
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Dysgonomonas sp900079735 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Dysgonomonadaceae; Dysgonomonas; Dysgonomonas sp900079735 | |||||||||||
| CAZyme ID | MGYG000003362_01852 | |||||||||||
| CAZy Family | GH20 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 81517; End: 84024 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH20 | 313 | 725 | 9.3e-92 | 0.9554896142433235 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06569 | GH20_Sm-chitobiase-like | 0.0 | 308 | 733 | 1 | 425 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| cd06563 | GH20_chitobiase-like | 3.25e-109 | 313 | 731 | 2 | 352 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This GH20 domain family includes an N-acetylglucosamidase (GlcNAcase A) from Pseudoalteromonas piscicida and an N-acetylhexosaminidase (SpHex) from Streptomyces plicatus. SpHex lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| pfam00728 | Glyco_hydro_20 | 1.05e-104 | 313 | 723 | 2 | 343 | Glycosyl hydrolase family 20, catalytic domain. This domain has a TIM barrel fold. |
| COG3525 | Chb | 9.17e-93 | 181 | 809 | 121 | 689 | N-acetyl-beta-hexosaminidase [Carbohydrate transport and metabolism]. |
| cd06568 | GH20_SpHex_like | 2.20e-53 | 313 | 731 | 2 | 324 | A subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the N-acetylhexosaminidase from Streptomyces plicatus (SpHex). SpHex catalyzes the hydrolysis of N-acetyl-beta-hexosaminides. An Asp residue within the active site plays a critical role in substrate-assisted catalysis by orienting the 2-acetamido group and stabilizing the transition state. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. Proteins belonging to this subgroup lack the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT73869.1 | 0.0 | 1 | 829 | 1 | 831 |
| CBK66887.1 | 2.23e-314 | 17 | 831 | 10 | 828 |
| QRN00147.1 | 3.07e-314 | 17 | 831 | 19 | 837 |
| QUT25549.1 | 6.16e-314 | 17 | 831 | 19 | 837 |
| QDH52947.1 | 6.16e-314 | 17 | 831 | 19 | 837 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1QBA_A | 1.08e-92 | 47 | 797 | 42 | 823 | BACTERIALCHITOBIASE, GLYCOSYL HYDROLASE FAMILY 20 [Serratia marcescens],1QBB_A BACTERIAL CHITOBIASE COMPLEXED WITH CHITOBIOSE (DINAG) [Serratia marcescens] |
| 1C7T_A | 2.08e-92 | 47 | 797 | 42 | 823 | ChainA, BETA-N-ACETYLHEXOSAMINIDASE [Serratia marcescens] |
| 1C7S_A | 1.07e-91 | 47 | 797 | 42 | 823 | ChainA, BETA-N-ACETYLHEXOSAMINIDASE [Serratia marcescens] |
| 6EZR_A | 1.69e-63 | 192 | 729 | 142 | 616 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZR_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZS_A Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6EZS_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6K35_A Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi],6K35_B Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi] |
| 6EZT_A | 2.02e-62 | 192 | 729 | 139 | 613 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi],6EZT_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q04786 | 1.25e-99 | 44 | 818 | 41 | 841 | Beta-hexosaminidase OS=Vibrio vulnificus OX=672 GN=hex PE=3 SV=1 |
| P13670 | 1.90e-96 | 50 | 795 | 71 | 846 | N,N'-diacetylchitobiase OS=Vibrio harveyi OX=669 GN=chb PE=1 SV=1 |
| Q54468 | 1.37e-91 | 47 | 797 | 69 | 850 | Chitobiase OS=Serratia marcescens OX=615 GN=chb PE=1 SV=1 |
| P49007 | 2.02e-88 | 43 | 730 | 54 | 732 | Beta-hexosaminidase B OS=Pseudoalteromonas piscicida OX=43662 GN=nag096 PE=3 SV=1 |
| P96155 | 2.04e-58 | 192 | 725 | 141 | 607 | Beta-hexosaminidase OS=Vibrio furnissii OX=29494 GN=exoI PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
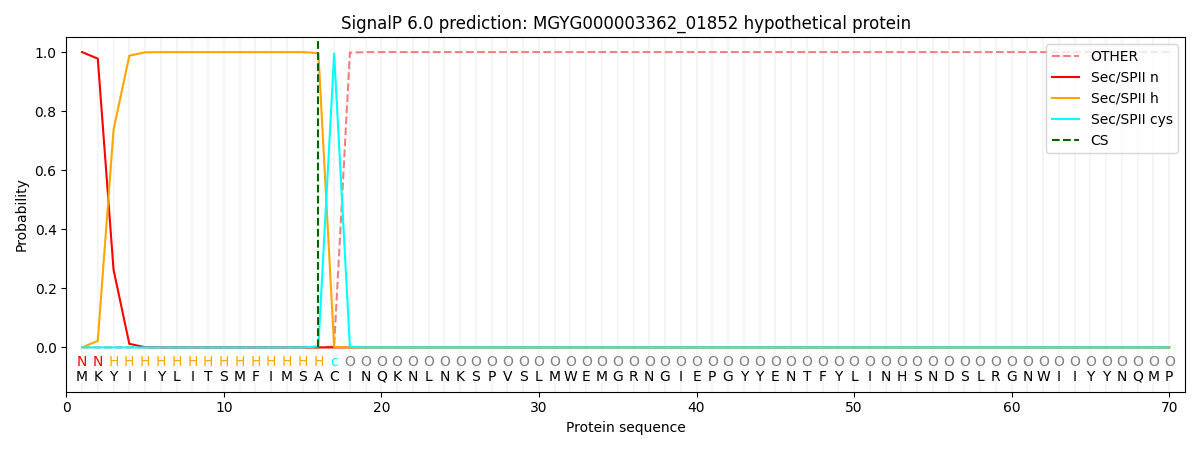
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000000 | 1.000036 | 0.000000 | 0.000000 | 0.000000 |
