You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003396_02967
You are here: Home > Sequence: MGYG000003396_02967
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
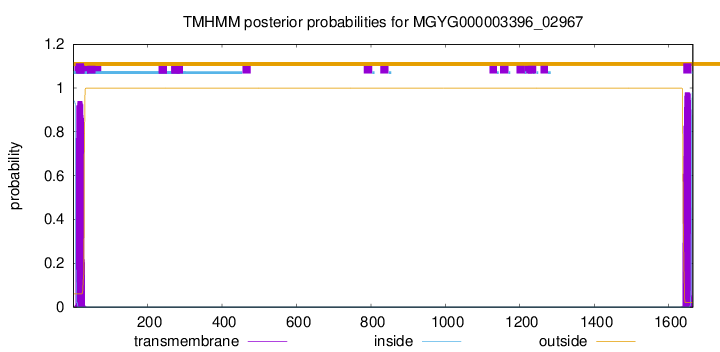
TMHMM annotations
Basic Information help
| Species | Clostridium sp900766315 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Clostridiales; Clostridiaceae; Clostridium; Clostridium sp900766315 | |||||||||||
| CAZyme ID | MGYG000003396_02967 | |||||||||||
| CAZy Family | CBM32 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 7447; End: 12444 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 377 | 809 | 2e-98 | 0.9678362573099415 |
| CBM40 | 191 | 358 | 8.4e-41 | 0.9720670391061452 |
| CBM40 | 964 | 1131 | 1.2e-38 | 0.9497206703910615 |
| CBM32 | 54 | 176 | 7.9e-19 | 0.9193548387096774 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd15482 | Sialidase_non-viral | 2.36e-85 | 380 | 811 | 2 | 339 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| COG4409 | NanH | 4.71e-30 | 290 | 801 | 187 | 703 | Neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| pfam02973 | Sialidase | 5.56e-26 | 184 | 361 | 1 | 188 | Sialidase, N-terminal domain. |
| pfam02973 | Sialidase | 1.86e-21 | 954 | 1132 | 1 | 188 | Sialidase, N-terminal domain. |
| pfam13088 | BNR_2 | 1.69e-15 | 556 | 795 | 79 | 280 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ATD57534.1 | 0.0 | 1 | 1665 | 1 | 1299 |
| QBJ75069.1 | 0.0 | 1 | 1665 | 1 | 1299 |
| ATD54786.1 | 0.0 | 1 | 1665 | 1 | 1299 |
| SLK16343.1 | 0.0 | 1 | 1665 | 1 | 1299 |
| AYE33273.1 | 3.38e-312 | 1 | 1525 | 1 | 1222 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5TSP_A | 1.29e-191 | 374 | 816 | 6 | 448 | Crystalstructure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124],5TSP_B Crystal structure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124] |
| 2BF6_A | 1.74e-190 | 374 | 816 | 5 | 447 | AtomicResolution Structure of the bacterial sialidase NanI from Clostridium perfringens in complex with alpha-Sialic Acid (Neu5Ac). [Clostridium perfringens] |
| 2VK5_A | 1.94e-190 | 374 | 816 | 5 | 447 | TheStructure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK6_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_B The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens] |
| 1SLI_A | 1.25e-86 | 180 | 818 | 1 | 668 | LeechIntramolecular Trans-Sialidase Complexed With Dana [Macrobdella decora],1SLL_A Sialidase L From Leech Macrobdella Decora [Macrobdella decora],2SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2,7- Anhydro-Neu5ac, The Reaction Product [Macrobdella decora],3SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2,7- Anhydro-Neu5ac Prepared By Soaking With 3'-Sialyllactose [Macrobdella decora],4SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2- Propenyl-Neu5ac, An Inactive Substrate Analogue [Macrobdella decora] |
| 2VVZ_A | 2.06e-81 | 376 | 831 | 6 | 480 | Structureof the catalytic domain of Streptococcus pneumoniae sialidase NanA [Streptococcus pneumoniae],2VVZ_B Structure of the catalytic domain of Streptococcus pneumoniae sialidase NanA [Streptococcus pneumoniae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P29767 | 4.36e-298 | 1 | 930 | 1 | 937 | Sialidase OS=Clostridium septicum OX=1504 PE=3 SV=1 |
| P62576 | 1.51e-85 | 182 | 892 | 116 | 849 | Sialidase A OS=Streptococcus pneumoniae (strain ATCC BAA-255 / R6) OX=171101 GN=nanA PE=1 SV=1 |
| P62575 | 1.51e-85 | 182 | 892 | 116 | 849 | Sialidase A OS=Streptococcus pneumoniae OX=1313 GN=nanA PE=1 SV=1 |
| Q27701 | 1.79e-85 | 173 | 818 | 74 | 748 | Anhydrosialidase OS=Macrobdella decora OX=6405 PE=1 SV=1 |
| Q54727 | 8.08e-38 | 197 | 811 | 51 | 682 | Sialidase B OS=Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334 / TIGR4) OX=170187 GN=nanB PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
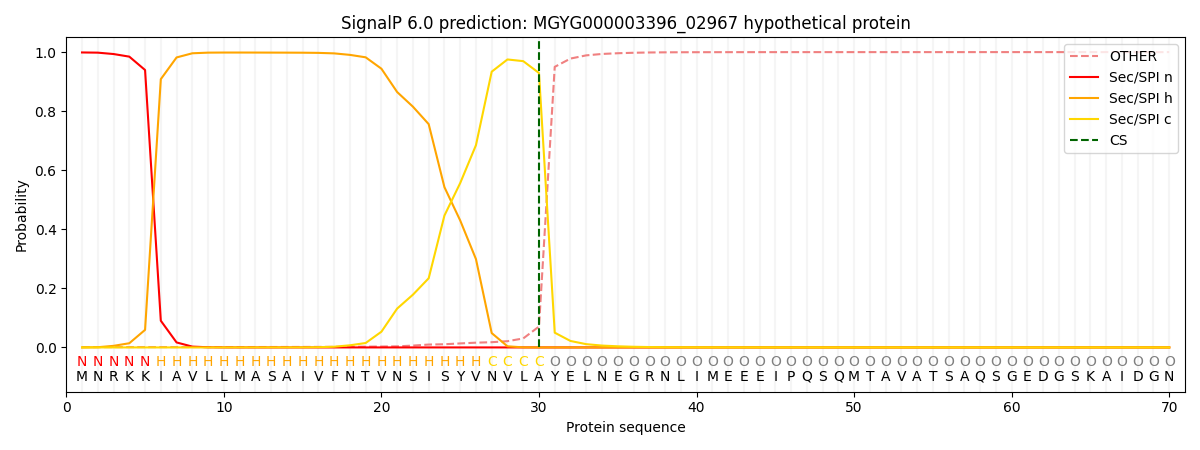
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002124 | 0.997004 | 0.000233 | 0.000240 | 0.000182 | 0.000168 |