You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003406_01168
You are here: Home > Sequence: MGYG000003406_01168
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Veillonella sp009929605 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_C; Negativicutes; Veillonellales; Veillonellaceae; Veillonella; Veillonella sp009929605 | |||||||||||
| CAZyme ID | MGYG000003406_01168 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2621; End: 4294 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH18 | 164 | 505 | 2.6e-28 | 0.7905405405405406 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd02874 | GH18_CFLE_spore_hydrolase | 1.58e-49 | 147 | 511 | 22 | 313 | Cortical fragment-lytic enzyme (CFLE) is a peptidoglycan hydrolase involved in bacterial endospore germination. CFLE is expressed as an inactive preprotein (called SleB) in the forespore compartment of sporulating cells. SleB translocates across the forespore inner membrane and is deposited as a mature enzyme in the cortex layer of the spore. As part of a sensory mechanism capable of initiating germination, CFLE degrades a spore-specific peptidoglycan constituent called muramic-acid delta-lactam that comprises the outer cortex. CFLE has a C-terminal glycosyl hydrolase family 18 (GH18) catalytic domain as well as two N-terminal LysM peptidoglycan-binding domains. In addition to SleB, this family includes YaaH, YdhD, and YvbX from Bacillus subtilis. |
| COG3858 | YaaH | 4.47e-33 | 145 | 512 | 120 | 419 | Spore germination protein YaaH [Cell cycle control, cell division, chromosome partitioning]. |
| smart00636 | Glyco_18 | 2.62e-16 | 146 | 351 | 36 | 249 | Glyco_18 domain. |
| pfam00704 | Glyco_hydro_18 | 2.45e-15 | 201 | 392 | 80 | 289 | Glycosyl hydrolases family 18. |
| cd02875 | GH18_chitobiase | 3.21e-12 | 204 | 509 | 91 | 340 | Chitobiase (also known as di-N-acetylchitobiase) is a lysosomal glycosidase that hydrolyzes the reducing-end N-acetylglucosamine from the chitobiose core of oligosaccharides during the ordered degradation of asparagine-linked glycoproteins in eukaryotes. Chitobiase can only do so if the asparagine that joins the oligosaccharide to protein is previously removed by a glycosylasparaginase. Chitobiase is therefore the final step in the lysosomal degradation of the protein/carbohydrate linkage component of asparagine-linked glycoproteins. The catalytic domain of chitobiase is an eight-stranded alpha/beta barrel fold similar to that of other family 18 glycosyl hydrolases such as hevamine and chitotriosidase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBU36194.1 | 1.20e-163 | 1 | 517 | 1 | 440 |
| SNU97803.1 | 2.82e-163 | 1 | 517 | 1 | 440 |
| QQB17876.1 | 2.82e-163 | 1 | 517 | 1 | 440 |
| ACZ24646.1 | 2.82e-163 | 1 | 517 | 1 | 440 |
| ARF99299.1 | 2.88e-163 | 1 | 517 | 1 | 440 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3CZ8_A | 2.50e-13 | 190 | 344 | 76 | 230 | ChainA, Putative sporulation-specific glycosylase ydhD [Bacillus subtilis subsp. subtilis str. 168],3CZ8_B Chain B, Putative sporulation-specific glycosylase ydhD [Bacillus subtilis subsp. subtilis str. 168] |
| 1WNO_A | 8.02e-06 | 225 | 350 | 130 | 273 | Crystalstructure of a native chitinase from Aspergillus fumigatus YJ-407 [Aspergillus fumigatus],1WNO_B Crystal structure of a native chitinase from Aspergillus fumigatus YJ-407 [Aspergillus fumigatus] |
| 1W9P_A | 8.64e-06 | 225 | 350 | 168 | 311 | Specificityand affinity of natural product cyclopentapeptide inhibitors against Aspergillus fumigatus, human and bacterial chitinaseFra [Aspergillus fumigatus],1W9P_B Specificity and affinity of natural product cyclopentapeptide inhibitors against Aspergillus fumigatus, human and bacterial chitinaseFra [Aspergillus fumigatus],1W9U_A Specificity and affnity of natural product cyclopentapeptide inhibitor Argadin against Aspergillus fumigatus chitinase [Aspergillus fumigatus],1W9U_B Specificity and affnity of natural product cyclopentapeptide inhibitor Argadin against Aspergillus fumigatus chitinase [Aspergillus fumigatus],1W9V_A Specificity and affinity of natural product cyclopentapeptide argifin against Aspergillus fumigatus [Aspergillus fumigatus],1W9V_B Specificity and affinity of natural product cyclopentapeptide argifin against Aspergillus fumigatus [Aspergillus fumigatus],2A3A_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with theophylline [Aspergillus fumigatus],2A3A_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with theophylline [Aspergillus fumigatus],2A3B_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with caffeine [Aspergillus fumigatus],2A3B_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with caffeine [Aspergillus fumigatus],2A3C_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with pentoxifylline [Aspergillus fumigatus],2A3C_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with pentoxifylline [Aspergillus fumigatus],2A3E_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with allosamidin [Aspergillus fumigatus],2A3E_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with allosamidin [Aspergillus fumigatus],2IUZ_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with C2-dicaffeine [Aspergillus fumigatus],2IUZ_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with C2-dicaffeine [Aspergillus fumigatus],3CH9_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with dimethylguanylurea [Aspergillus fumigatus],3CH9_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with dimethylguanylurea [Aspergillus fumigatus],3CHC_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with monopeptide [Aspergillus fumigatus],3CHC_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with monopeptide [Aspergillus fumigatus],3CHD_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with dipeptide [Aspergillus fumigatus],3CHD_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with dipeptide [Aspergillus fumigatus],3CHE_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with tripeptide [Aspergillus fumigatus],3CHE_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with tripeptide [Aspergillus fumigatus],3CHF_A Crystal structure of Aspergillus fumigatus chitinase B1 in complex with tetrapeptide [Aspergillus fumigatus],3CHF_B Crystal structure of Aspergillus fumigatus chitinase B1 in complex with tetrapeptide [Aspergillus fumigatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O32258 | 1.17e-19 | 186 | 349 | 86 | 257 | Uncharacterized glycosylase YvbX OS=Bacillus subtilis (strain 168) OX=224308 GN=yvbX PE=3 SV=1 |
| O31682 | 9.23e-19 | 152 | 356 | 27 | 224 | Putative glycosylase YkvQ OS=Bacillus subtilis (strain 168) OX=224308 GN=ykvQ PE=3 SV=1 |
| O05495 | 2.73e-12 | 190 | 344 | 168 | 322 | Putative sporulation-specific glycosylase YdhD OS=Bacillus subtilis (strain 168) OX=224308 GN=ydhD PE=1 SV=2 |
| P37531 | 3.23e-08 | 191 | 380 | 173 | 367 | Cortical fragment-lytic enzyme OS=Bacillus subtilis (strain 168) OX=224308 GN=sleL PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
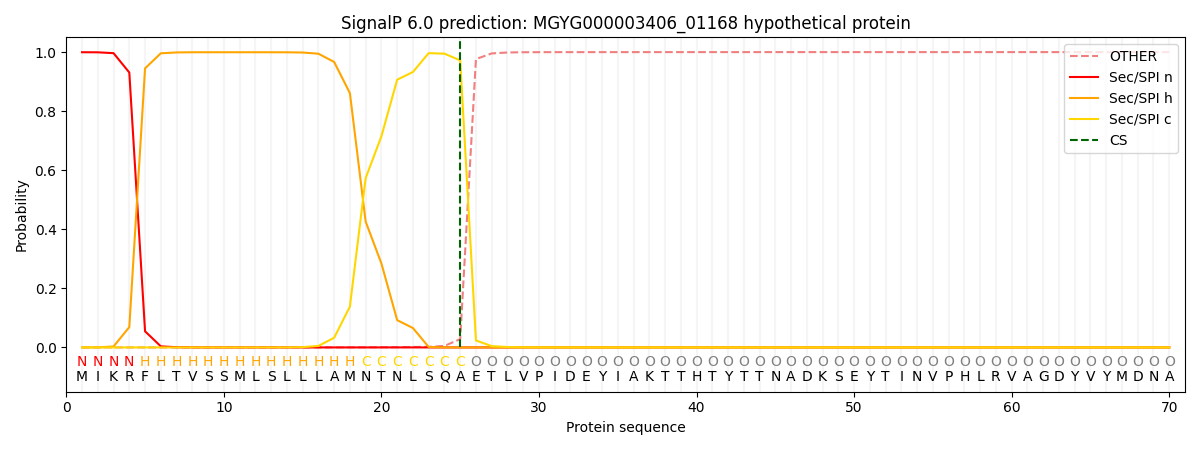
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000421 | 0.997808 | 0.001117 | 0.000230 | 0.000209 | 0.000190 |
