You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003461_01238
You are here: Home > Sequence: MGYG000003461_01238
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp900553595 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900553595 | |||||||||||
| CAZyme ID | MGYG000003461_01238 | |||||||||||
| CAZy Family | GH95 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8480; End: 10948 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH95 | 56 | 797 | 9.9e-267 | 0.9778393351800554 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam14498 | Glyco_hyd_65N_2 | 1.68e-54 | 57 | 307 | 4 | 233 | Glycosyl hydrolase family 65, N-terminal domain. This domain represents a domain found to the N-terminus of the glycosyl hydrolase 65 family catalytic domain. |
| pfam03632 | Glyco_hydro_65m | 1.73e-04 | 515 | 610 | 125 | 229 | Glycosyl hydrolase family 65 central catalytic domain. This family of glycosyl hydrolases contains vacuolar acid trehalase and maltose phosphorylase.Maltose phosphorylase (MP) is a dimeric enzyme that catalyzes the conversion of maltose and inorganic phosphate into beta-D-glucose-1-phosphate and glucose. The central domain is the catalytic domain, which binds a phosphate ion that is proximal the the highly conserved Glu. The arrangement of the phosphate and the glutamate is thought to cause nucleophilic attack on the anomeric carbon atom. The catalytic domain also forms the majority of the dimerization interface. |
| COG1554 | ATH1 | 4.77e-04 | 515 | 610 | 443 | 548 | Trehalose and maltose hydrolase (possible phosphorylase) [Carbohydrate transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QKH85901.1 | 0.0 | 16 | 810 | 26 | 819 |
| QCQ35127.1 | 0.0 | 16 | 810 | 26 | 819 |
| QCQ48529.1 | 0.0 | 16 | 810 | 26 | 819 |
| QRP88070.1 | 0.0 | 16 | 810 | 26 | 819 |
| QTO26524.1 | 0.0 | 16 | 810 | 26 | 819 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4UFC_A | 1.06e-158 | 59 | 792 | 29 | 742 | Crystalstructure of the GH95 enzyme BACOVA_03438 [Bacteroides ovatus],4UFC_B Crystal structure of the GH95 enzyme BACOVA_03438 [Bacteroides ovatus] |
| 7KMQ_A | 3.79e-155 | 59 | 797 | 48 | 763 | ChainA, Glyco_hyd_65N_2 domain-containing protein [Xanthomonas citri pv. citri str. 306],7KMQ_B Chain B, Glyco_hyd_65N_2 domain-containing protein [Xanthomonas citri pv. citri str. 306] |
| 2RDY_A | 1.61e-151 | 59 | 816 | 10 | 795 | ChainA, BH0842 protein [Halalkalibacterium halodurans C-125],2RDY_B Chain B, BH0842 protein [Halalkalibacterium halodurans C-125] |
| 2EAB_A | 8.74e-141 | 34 | 792 | 20 | 850 | Crystalstructure of 1,2-a-L-fucosidase from Bifidobacterium bifidum (apo form) [Bifidobacterium bifidum],2EAB_B Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum (apo form) [Bifidobacterium bifidum],2EAC_A Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum in complex with deoxyfuconojirimycin [Bifidobacterium bifidum],2EAC_B Crystal structure of 1,2-a-L-fucosidase from Bifidobacterium bifidum in complex with deoxyfuconojirimycin [Bifidobacterium bifidum] |
| 2EAD_A | 6.68e-140 | 34 | 792 | 20 | 850 | ChainA, Alpha-fucosidase [Bifidobacterium bifidum],2EAD_B Chain B, Alpha-fucosidase [Bifidobacterium bifidum] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8L7W8 | 1.20e-139 | 25 | 776 | 33 | 797 | Alpha-L-fucosidase 2 OS=Arabidopsis thaliana OX=3702 GN=FUC95A PE=1 SV=1 |
| A2R797 | 9.06e-106 | 67 | 793 | 38 | 781 | Probable alpha-fucosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=afcA PE=3 SV=1 |
| Q5AU81 | 4.59e-77 | 70 | 772 | 46 | 776 | Alpha-fucosidase A OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=afcA PE=1 SV=1 |
| Q2USL3 | 3.96e-67 | 61 | 791 | 24 | 715 | Probable alpha-fucosidase A OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=afcA PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
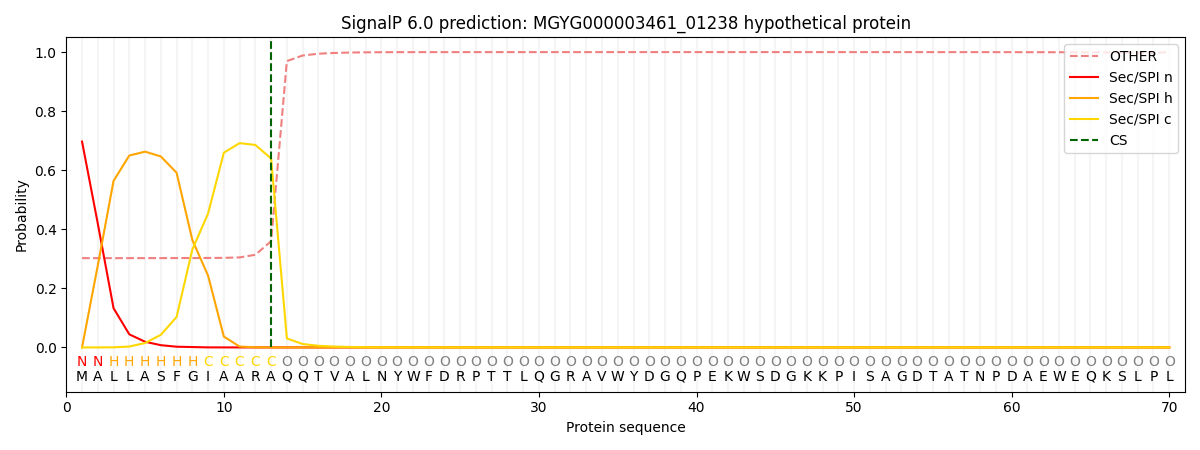
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.314871 | 0.682568 | 0.000791 | 0.001049 | 0.000403 | 0.000306 |
