You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003480_00519
You are here: Home > Sequence: MGYG000003480_00519
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; | |||||||||||
| CAZyme ID | MGYG000003480_00519 | |||||||||||
| CAZy Family | GH20 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1545; End: 3917 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH20 | 170 | 525 | 6.5e-125 | 0.973293768545994 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06563 | GH20_chitobiase-like | 0.0 | 175 | 538 | 1 | 357 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This GH20 domain family includes an N-acetylglucosamidase (GlcNAcase A) from Pseudoalteromonas piscicida and an N-acetylhexosaminidase (SpHex) from Streptomyces plicatus. SpHex lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| pfam00728 | Glyco_hydro_20 | 6.90e-175 | 175 | 525 | 1 | 345 | Glycosyl hydrolase family 20, catalytic domain. This domain has a TIM barrel fold. |
| COG3525 | Chb | 2.12e-133 | 114 | 627 | 200 | 712 | N-acetyl-beta-hexosaminidase [Carbohydrate transport and metabolism]. |
| cd06568 | GH20_SpHex_like | 4.32e-103 | 175 | 538 | 1 | 329 | A subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the N-acetylhexosaminidase from Streptomyces plicatus (SpHex). SpHex catalyzes the hydrolysis of N-acetyl-beta-hexosaminides. An Asp residue within the active site plays a critical role in substrate-assisted catalysis by orienting the 2-acetamido group and stabilizing the transition state. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. Proteins belonging to this subgroup lack the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. |
| cd06570 | GH20_chitobiase-like_1 | 9.63e-86 | 175 | 538 | 1 | 311 | A functionally uncharacterized subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the chitobiase of Serratia marcescens, a beta-N-1,4-acetylhexosaminidase that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This subgroup lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QRP57800.1 | 0.0 | 21 | 789 | 8 | 773 |
| QUU08752.1 | 0.0 | 21 | 789 | 8 | 773 |
| ASM66469.1 | 0.0 | 21 | 789 | 8 | 773 |
| QQT76952.1 | 0.0 | 21 | 789 | 8 | 773 |
| QRO26332.1 | 0.0 | 23 | 787 | 13 | 774 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6Q63_A | 0.0 | 21 | 789 | 8 | 773 | BT0459[Bacteroides thetaiotaomicron],6Q63_B BT0459 [Bacteroides thetaiotaomicron],6Q63_C BT0459 [Bacteroides thetaiotaomicron] |
| 3RCN_A | 3.66e-123 | 106 | 538 | 65 | 498 | CrystalStructure of Beta-N-Acetylhexosaminidase from Arthrobacter aurescens [Paenarthrobacter aurescens TC1] |
| 7DUP_A | 2.56e-116 | 88 | 560 | 49 | 528 | ChainA, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVA_A Chain A, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVA_B Chain B, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron] |
| 7DVB_A | 1.40e-115 | 88 | 560 | 49 | 528 | ChainA, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_B Chain B, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_C Chain C, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_D Chain D, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron] |
| 7CBN_A | 8.68e-115 | 43 | 554 | 12 | 512 | Crystalstructure of beta-N-acetylhexosaminidase Am0868 from Akkermansia muciniphila [Akkermansia muciniphila ATCC BAA-835],7CBO_A Crystal structure of beta-N-acetylhexosaminidase Am0868 from Akkermansia muciniphila in complex with GlcNAc [Akkermansia muciniphila ATCC BAA-835] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P49008 | 1.28e-150 | 25 | 601 | 8 | 582 | Beta-hexosaminidase OS=Porphyromonas gingivalis (strain ATCC BAA-308 / W83) OX=242619 GN=nahA PE=3 SV=2 |
| B2UQG6 | 6.60e-114 | 43 | 554 | 31 | 531 | Beta-hexosaminidase Amuc_0868 OS=Akkermansia muciniphila (strain ATCC BAA-835 / DSM 22959 / JCM 33894 / BCRC 81048 / CCUG 64013 / CIP 107961 / Muc) OX=349741 GN=Amuc_0868 PE=1 SV=1 |
| P96155 | 1.05e-87 | 40 | 519 | 137 | 601 | Beta-hexosaminidase OS=Vibrio furnissii OX=29494 GN=exoI PE=1 SV=1 |
| B2UP57 | 7.04e-72 | 105 | 540 | 41 | 464 | Beta-hexosaminidase Amuc_2018 OS=Akkermansia muciniphila (strain ATCC BAA-835 / DSM 22959 / JCM 33894 / BCRC 81048 / CCUG 64013 / CIP 107961 / Muc) OX=349741 GN=Amuc_2018 PE=1 SV=1 |
| Q7WUL4 | 2.74e-67 | 41 | 552 | 3 | 477 | Beta-N-acetylhexosaminidase OS=Cellulomonas fimi OX=1708 GN=hex20 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
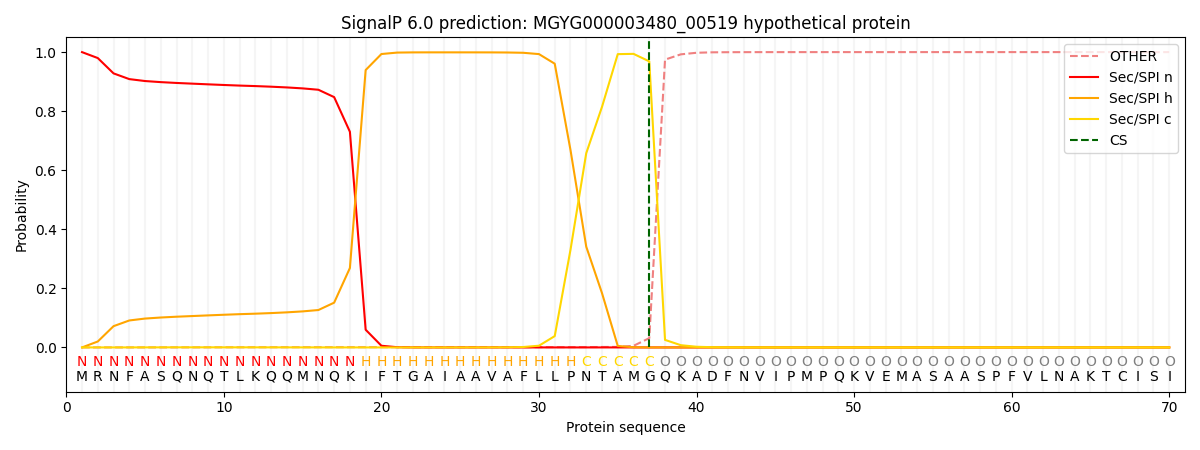
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000563 | 0.998670 | 0.000184 | 0.000224 | 0.000182 | 0.000158 |

