You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003575_01876
You are here: Home > Sequence: MGYG000003575_01876
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Treponema_D sp900770075 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Spirochaetota; Spirochaetia; Treponematales; Treponemataceae; Treponema_D; Treponema_D sp900770075 | |||||||||||
| CAZyme ID | MGYG000003575_01876 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | Pectate lyase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1370; End: 2680 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 166 | 369 | 3.3e-66 | 0.971830985915493 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00656 | Amb_all | 1.77e-50 | 175 | 369 | 16 | 186 | Amb_all domain. |
| COG3866 | PelB | 2.79e-47 | 33 | 432 | 35 | 344 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam00544 | Pec_lyase_C | 4.14e-36 | 175 | 369 | 34 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AEJ18226.1 | 3.45e-121 | 24 | 436 | 53 | 475 |
| AEE17959.1 | 8.92e-85 | 21 | 434 | 42 | 446 |
| QPH91275.1 | 1.97e-66 | 25 | 436 | 24 | 416 |
| QPI07285.1 | 2.31e-64 | 6 | 436 | 3 | 416 |
| QPH96000.1 | 1.77e-63 | 6 | 436 | 3 | 416 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1VBL_A | 5.30e-51 | 33 | 432 | 12 | 415 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
| 5AMV_A | 7.57e-49 | 32 | 428 | 11 | 396 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
| 1BN8_A | 1.20e-48 | 32 | 428 | 32 | 417 | BacillusSubtilis Pectate Lyase [Bacillus subtilis] |
| 2BSP_A | 3.27e-48 | 32 | 428 | 32 | 417 | ChainA, PROTEIN (PECTATE LYASE) [Bacillus subtilis] |
| 2NZM_A | 5.60e-48 | 32 | 428 | 11 | 396 | ChainA, Pectate lyase [Bacillus subtilis],2O04_A Chain A, Pectate lyase [Bacillus subtilis],2O0V_A Chain A, Pectate lyase [Bacillus subtilis],2O0W_A Chain A, Pectate lyase [Bacillus subtilis],2O17_A Chain A, Pectate lyase [Bacillus subtilis],2O1D_A Chain A, Pectate lyase [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P39116 | 6.59e-48 | 32 | 428 | 32 | 417 | Pectate lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pel PE=1 SV=1 |
| P04960 | 1.32e-41 | 171 | 432 | 106 | 385 | Pectate lyase E OS=Dickeya chrysanthemi OX=556 GN=pelE PE=1 SV=1 |
| P18209 | 3.97e-38 | 171 | 432 | 112 | 391 | Pectate lyase D OS=Dickeya chrysanthemi OX=556 GN=pelD PE=3 SV=1 |
| P0C1A4 | 7.05e-38 | 171 | 432 | 124 | 404 | Pectate lyase E OS=Dickeya chrysanthemi OX=556 GN=pelE PE=3 SV=1 |
| P0C1A5 | 7.05e-38 | 171 | 432 | 124 | 404 | Pectate lyase E OS=Dickeya dadantii (strain 3937) OX=198628 GN=pelE PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
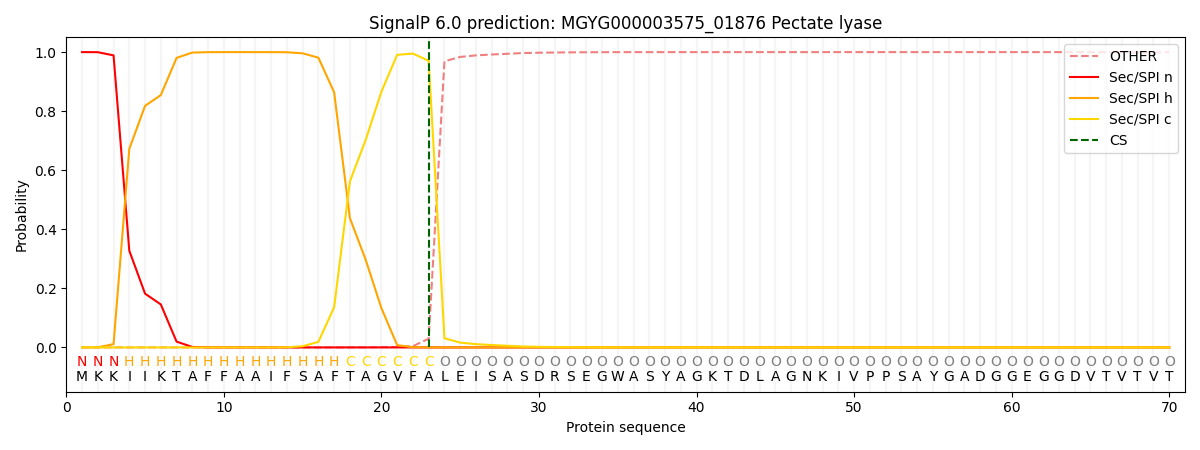
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001294 | 0.997280 | 0.000375 | 0.000398 | 0.000340 | 0.000287 |

