You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003622_01780
You are here: Home > Sequence: MGYG000003622_01780
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp900770845 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900770845 | |||||||||||
| CAZyme ID | MGYG000003622_01780 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8291; End: 9673 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 24 | 371 | 1.4e-105 | 0.9508670520231214 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam01120 | Alpha_L_fucos | 2.98e-118 | 34 | 368 | 2 | 333 | Alpha-L-fucosidase. |
| smart00812 | Alpha_L_fucos | 1.06e-102 | 32 | 399 | 1 | 370 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
| COG3669 | AfuC | 5.36e-44 | 63 | 374 | 1 | 323 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADV43482.1 | 2.67e-241 | 1 | 460 | 1 | 457 |
| QRO24330.1 | 3.06e-235 | 20 | 460 | 19 | 462 |
| ADV43484.1 | 7.18e-233 | 13 | 460 | 11 | 461 |
| QQY39446.1 | 1.91e-228 | 20 | 460 | 18 | 462 |
| QEW35132.1 | 1.91e-228 | 20 | 460 | 18 | 462 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6GN6_A | 2.10e-56 | 57 | 371 | 38 | 352 | Alpha-L-fucosidaseisoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_B Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_C Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_D Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_E Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_F Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus] |
| 4PCS_A | 5.74e-41 | 50 | 399 | 19 | 376 | Crystalstructure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_B Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_C Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_D Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_A Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_B Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_C Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_D Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482] |
| 2WVT_A | 6.16e-41 | 50 | 399 | 23 | 380 | Crystalstructure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron in complex with a novel iminosugar fucosidase inhibitor [Bacteroides thetaiotaomicron VPI-5482],2WVT_B Crystal structure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron in complex with a novel iminosugar fucosidase inhibitor [Bacteroides thetaiotaomicron VPI-5482],2WVU_A Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_B Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_C Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_D Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482] |
| 4WSJ_A | 6.39e-41 | 50 | 399 | 19 | 376 | Crystalstructure of a bacterial fucodiase in complex with 1-((1R,2R,3R,4R,5R,6R)-2,3,4-trihydroxy-5-methyl-7-azabicyclo[4.1.0]heptan-7-yl)ethan-1-one [Bacteroides thetaiotaomicron VPI-5482],4WSJ_B Crystal structure of a bacterial fucodiase in complex with 1-((1R,2R,3R,4R,5R,6R)-2,3,4-trihydroxy-5-methyl-7-azabicyclo[4.1.0]heptan-7-yl)ethan-1-one [Bacteroides thetaiotaomicron VPI-5482],4WSJ_C Crystal structure of a bacterial fucodiase in complex with 1-((1R,2R,3R,4R,5R,6R)-2,3,4-trihydroxy-5-methyl-7-azabicyclo[4.1.0]heptan-7-yl)ethan-1-one [Bacteroides thetaiotaomicron VPI-5482],4WSJ_D Crystal structure of a bacterial fucodiase in complex with 1-((1R,2R,3R,4R,5R,6R)-2,3,4-trihydroxy-5-methyl-7-azabicyclo[4.1.0]heptan-7-yl)ethan-1-one [Bacteroides thetaiotaomicron VPI-5482] |
| 5I5R_A | 6.50e-41 | 50 | 399 | 20 | 377 | Crystalstructure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],5I5R_B Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],5I5R_C Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],5I5R_D Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q99KR8 | 1.54e-37 | 48 | 399 | 38 | 393 | Plasma alpha-L-fucosidase OS=Mus musculus OX=10090 GN=Fuca2 PE=1 SV=1 |
| Q6AYS4 | 2.84e-37 | 48 | 399 | 36 | 391 | Plasma alpha-L-fucosidase OS=Rattus norvegicus OX=10116 GN=Fuca2 PE=2 SV=1 |
| Q9BTY2 | 4.43e-37 | 48 | 399 | 44 | 399 | Plasma alpha-L-fucosidase OS=Homo sapiens OX=9606 GN=FUCA2 PE=1 SV=2 |
| Q5RFI5 | 2.13e-36 | 48 | 399 | 42 | 397 | Plasma alpha-L-fucosidase OS=Pongo abelii OX=9601 GN=FUCA2 PE=2 SV=1 |
| C3YWU0 | 2.98e-35 | 48 | 450 | 28 | 433 | Alpha-L-fucosidase OS=Branchiostoma floridae OX=7739 GN=BRAFLDRAFT_56888 PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
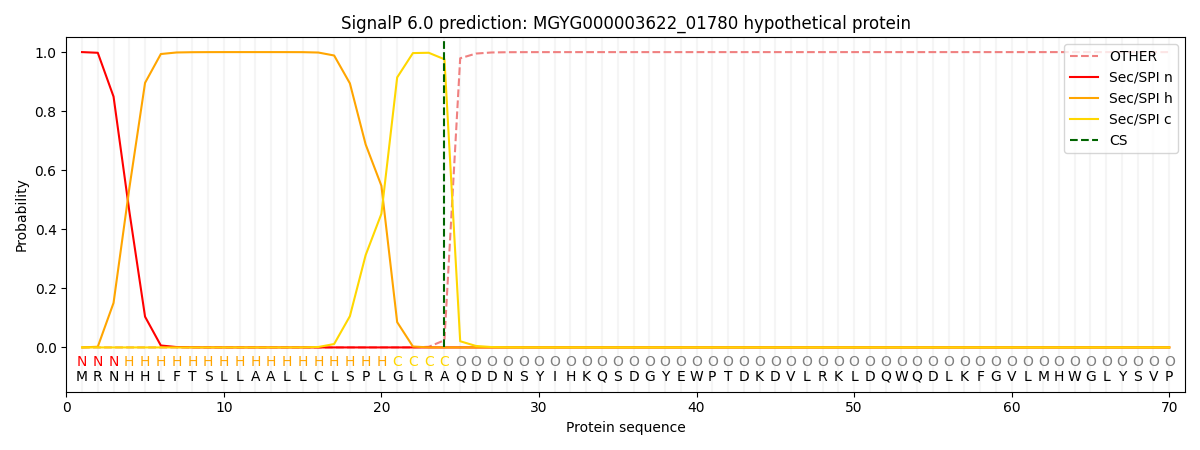
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000329 | 0.998795 | 0.000268 | 0.000206 | 0.000196 | 0.000181 |
