You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003668_01113
You are here: Home > Sequence: MGYG000003668_01113
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
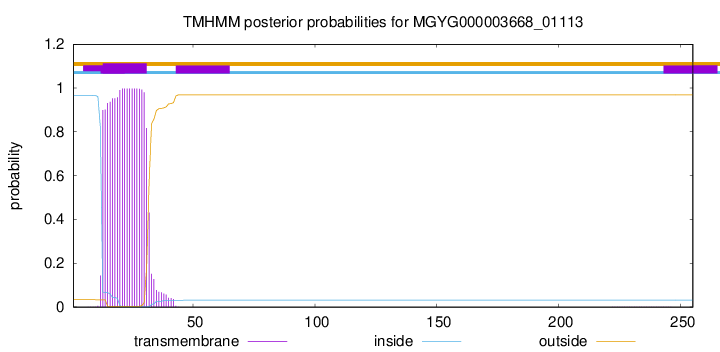
TMHMM annotations
Basic Information help
| Species | UBA1777 sp900549485 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Oscillospiraceae; UBA1777; UBA1777 sp900549485 | |||||||||||
| CAZyme ID | MGYG000003668_01113 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 22121; End: 22888 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 50 | 174 | 8.8e-35 | 0.9384615384615385 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10917 | CE4_NodB_like_6s_7s | 8.69e-75 | 56 | 226 | 1 | 171 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| TIGR02764 | spore_ybaN_pdaB | 2.53e-73 | 51 | 239 | 1 | 191 | polysaccharide deacetylase family sporulation protein PdaB. This model describes the YbaN protein family, also called PdaB and SpoVIE, of Gram-positive bacteria. Although ybaN null mutants have only a mild sporulation defect, ybaN/ytrI double mutants show drastically reducted sporulation efficiencies. This synthetic defect suggests the role of this sigmaE-controlled gene in sporulation had been masked by functional redundancy. Members of this family are homologous to a characterized polysaccharide deacetylase; the exact function this protein family is unknown. [Cellular processes, Sporulation and germination] |
| cd10950 | CE4_BsYlxY_like | 4.13e-68 | 53 | 239 | 3 | 188 | Putative catalytic NodB homology domain of uncharacterized protein YlxY from Bacillus subtilis and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis putative polysaccharide deacetylase BsYlxY, encoded by the ylxY gene, which is a member of the carbohydrate esterase 4 (CE4) superfamily. Although its biological function still remains unknown, BsYlxY shows high sequence homology to the catalytic domain of Bacillus subtilis pdaB gene encoding a putative polysaccharide deacetylase (BsPdaB), which is essential for the maintenance of spores after the late stage of sporulation and is highly conserved in spore-forming bacteria. However, disruption of the ylxY gene in B. subtilis did not cause any sporulation defect. Moreover, the Asp residue in the classical His-His-Asp zinc-binding motif of CE4 esterases is mutated to a Val residue in this family. Other catalytically relevant residues of CE4 esterases are also not conserved, which suggest that members of this family may be inactive. |
| cd10949 | CE4_BsPdaB_like | 5.84e-60 | 56 | 239 | 4 | 190 | Putative catalytic NodB homology domain of Bacillus subtilis putative polysaccharide deacetylase PdaB, and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by the putative polysaccharide deacetylase PdaB encoded by the pdaB gene on sporulation of Bacillus subtilis. Although its biochemical properties remain to be determined, the PdaB (YbaN) protein is essential for maintaining spores after the late stage of sporulation and is highly conserved in spore-forming bacteria. The glycans of the spore cortex may be candidate PdaB substrates. Based on sequence similarity, the family members are classified as carbohydrate esterase 4 (CE4) superfamily members. However, the classical His-His-Asp zinc-binding motif of CE4 esterases is missing in this family. |
| cd10954 | CE4_CtAXE_like | 5.47e-58 | 56 | 237 | 1 | 179 | Catalytic NodB homology domain of Clostridium thermocellum acetylxylan esterase and its bacterial homologs. This family is represented by Clostridium thermocellum acetylxylan esterase (CtAXE, EC 3.1.1.72), a member of the carbohydrate esterase 4 (CE4) superfamily. CtAXE deacetylates O-acetylated xylan, a key component of plant cell walls. It shows no detectable activity on generic esterase substrates including para-nitrophenyl acetate. It is specific for sugar-based substrates and will precipitate acetylxylan, as a consequence of deacetylation. CtAXE is a monomeric protein containing a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold as other CE4 esterases. However, due to differences in the topography of the substrate-binding groove, the chemistry of the active center, and metal ion coordination, CtAXE has different metal ion preference and lacks activity on N-acetyl substrates. It is significantly activated by Co2+. Moreover, CtAXE displays distinctly different ligand coordination to the metal ion, utilizing an aspartate, a histidine, and four water molecules, as opposed to the conserved His-His-Asp zinc-binding triad of other CE4 esterases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANU39676.1 | 4.49e-109 | 12 | 255 | 8 | 255 |
| QQR07498.1 | 4.49e-109 | 12 | 255 | 8 | 255 |
| QIA29235.1 | 4.49e-109 | 12 | 255 | 8 | 255 |
| QNL43472.1 | 7.31e-108 | 27 | 252 | 23 | 252 |
| BCK79020.1 | 1.36e-104 | 11 | 255 | 12 | 260 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7FBW_A | 5.23e-40 | 27 | 239 | 85 | 300 | ChainA, Predicted xylanase/chitin deacetylase [Caldanaerobacter subterraneus subsp. tengcongensis MB4] |
| 6HM9_A | 1.19e-38 | 47 | 239 | 76 | 267 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme with restored enzymatic activity. [Bacillus anthracis] |
| 6HPA_A | 4.13e-38 | 47 | 239 | 77 | 268 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme [Bacillus anthracis] |
| 7BKF_A | 1.01e-37 | 47 | 248 | 77 | 277 | ChainA, Putative polysaccharide deacetylase [Bacillus anthracis] |
| 6H8L_A | 2.93e-32 | 50 | 238 | 4 | 189 | Structureof peptidoglycan deacetylase PdaC from Bacillus subtilis [Bacillus subtilis subsp. subtilis str. 168],6H8L_B Structure of peptidoglycan deacetylase PdaC from Bacillus subtilis [Bacillus subtilis subsp. subtilis str. 168] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P50850 | 5.00e-36 | 47 | 239 | 121 | 312 | Uncharacterized protein YlxY OS=Bacillus subtilis (strain 168) OX=224308 GN=ylxY PE=3 SV=2 |
| P02963 | 1.00e-30 | 54 | 235 | 19 | 213 | Chitooligosaccharide deacetylase OS=Rhizobium meliloti (strain 1021) OX=266834 GN=nodB PE=3 SV=3 |
| Q8DP63 | 1.05e-29 | 49 | 239 | 261 | 448 | Peptidoglycan-N-acetylglucosamine deacetylase OS=Streptococcus pneumoniae (strain ATCC BAA-255 / R6) OX=171101 GN=pgdA PE=1 SV=1 |
| O34798 | 1.51e-29 | 50 | 238 | 272 | 457 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaC OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaC PE=1 SV=1 |
| A0A3Q0NBH7 | 1.03e-28 | 46 | 238 | 256 | 445 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain EGD / Mackaness) OX=1334565 GN=pgdA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
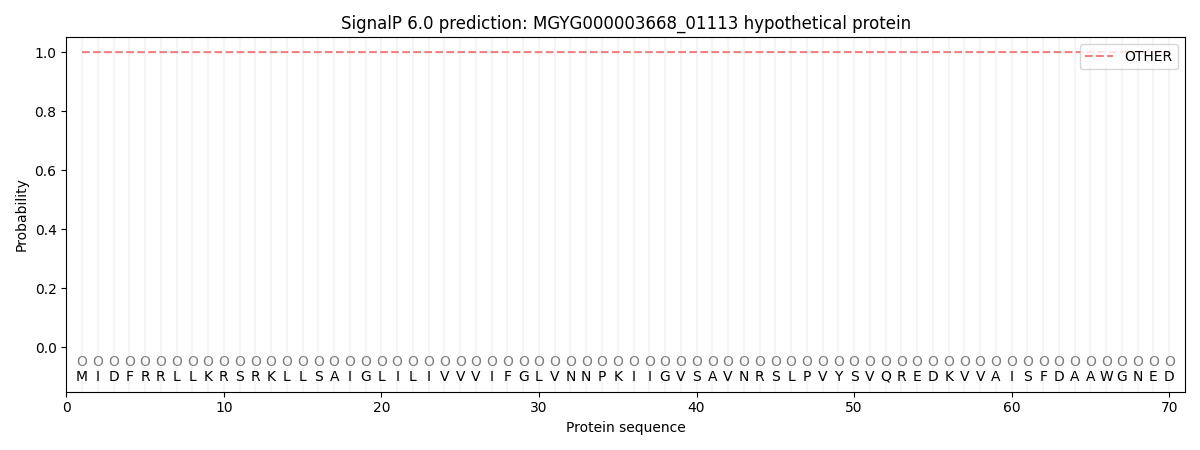
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.999875 | 0.000115 | 0.000011 | 0.000001 | 0.000000 | 0.000031 |