You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003719_00311
You are here: Home > Sequence: MGYG000003719_00311
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
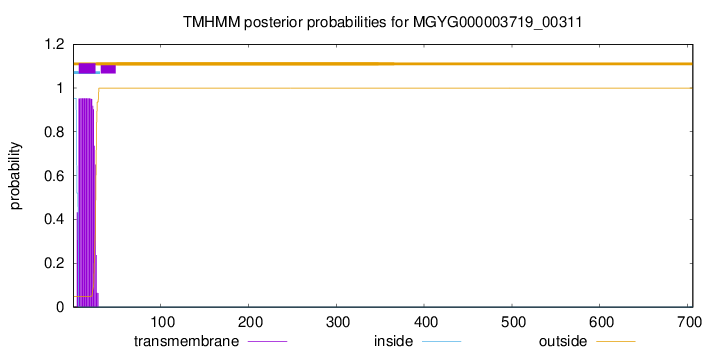
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Tissierellales; Peptoniphilaceae; Peptoniphilus_B; | |||||||||||
| CAZyme ID | MGYG000003719_00311 | |||||||||||
| CAZy Family | CBM50 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 34624; End: 36744 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG0737 | UshA | 3.78e-117 | 19 | 510 | 12 | 513 | 2',3'-cyclic-nucleotide 2'-phosphodiesterase/5'- or 3'-nucleotidase, 5'-nucleotidase family [Nucleotide transport and metabolism, Defense mechanisms]. |
| PRK09419 | PRK09419 | 2.06e-103 | 35 | 509 | 660 | 1144 | multifunctional 2',3'-cyclic-nucleotide 2'-phosphodiesterase/3'-nucleotidase/5'-nucleotidase. |
| cd07408 | MPP_SA0022_N | 1.06e-79 | 36 | 291 | 1 | 255 | Staphylococcus aureus SA0022 and related proteins, N-terminal metallophosphatase domain. SA0022 is an uncharacterized Staphylococcus aureus UshA-like protein with two putative domains, an N-terminal metallophosphatase domain and a C-terminal nucleotidase domain. SA0022 also contains a putative C-terminal cell wall anchor domain. The N-terminal metallophosphatase domain belongs to a large superfamily of distantly related metallophosphatases (MPPs) that includes: Mre11/SbcD-like exonucleases, Dbr1-like RNA lariat debranching enzymes, YfcE-like phosphodiesterases, purple acid phosphatases (PAPs), YbbF-like UDP-2,3-diacylglucosamine hydrolases, and acid sphingomyelinases (ASMases). MPPs are functionally diverse, but all share a conserved domain with an active site consisting of two metal ions (usually manganese, iron, or zinc) coordinated with octahedral geometry by a cage of histidine, aspartate, and asparagine residues. The conserved domain is a double beta-sheet sandwich with a di-metal active site made up of residues located at the C-terminal side of the sheets. This domain is thought to allow for productive metal coordination. |
| PRK09558 | ushA | 1.40e-79 | 17 | 501 | 18 | 538 | bifunctional UDP-sugar hydrolase/5'-nucleotidase periplasmic precursor; Reviewed |
| cd00845 | MPP_UshA_N_like | 1.26e-65 | 36 | 290 | 1 | 253 | Escherichia coli UshA-like family, N-terminal metallophosphatase domain. This family includes the bacterial enzyme UshA, and related enzymes including SoxB, CpdB, YhcR, and CD73. All members have a similar domain architecture which includes an N-terminal metallophosphatase domain and a C-terminal nucleotidase domain. The N-terminal metallophosphatase domain belongs to a large superfamily of distantly related metallophosphatases (MPPs) that includes: Mre11/SbcD-like exonucleases, Dbr1-like RNA lariat debranching enzymes, YfcE-like phosphodiesterases, purple acid phosphatases (PAPs), YbbF-like UDP-2,3-diacylglucosamine hydrolases, and acid sphingomyelinases (ASMases). MPPs are functionally diverse, but all share a conserved domain with an active site consisting of two metal ions (usually manganese, iron, or zinc) coordinated with octahedral geometry by a cage of histidine, aspartate, and asparagine residues. The conserved domain is a double beta-sheet sandwich with a di-metal active site made up of residues located at the C-terminal side of the sheets. This domain is thought to allow for productive metal coordination. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QQY79959.1 | 3.88e-163 | 24 | 510 | 29 | 510 |
| QZY54554.1 | 2.94e-159 | 1 | 521 | 1 | 516 |
| AGA59602.1 | 8.51e-148 | 21 | 510 | 21 | 504 |
| QOS77348.1 | 3.13e-144 | 10 | 520 | 9 | 518 |
| QLG42320.1 | 6.24e-144 | 10 | 520 | 9 | 518 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2Z1A_A | 4.45e-71 | 36 | 510 | 30 | 530 | Crystalstructure of 5'-nucleotidase precursor from Thermus thermophilus HB8 [Thermus thermophilus HB8] |
| 3IVD_A | 5.10e-57 | 35 | 506 | 6 | 494 | Putative5'-Nucleotidase (c4898) from Escherichia Coli in complex with Uridine [Escherichia coli O6],3IVD_B Putative 5'-Nucleotidase (c4898) from Escherichia Coli in complex with Uridine [Escherichia coli O6],3IVE_A Putative 5'-Nucleotidase (c4898) from Escherichia Coli in complex with Cytidine [Escherichia coli O6] |
| 4H2F_A | 1.70e-49 | 35 | 508 | 25 | 543 | Humanecto-5'-nucleotidase (CD73): crystal form I (open) in complex with adenosine [Homo sapiens],4H2G_A Human ecto-5'-nucleotidase (CD73): crystal form II (open) in complex with adenosine [Homo sapiens] |
| 4H1Y_P | 1.70e-49 | 35 | 508 | 25 | 543 | Humanecto-5'-nucleotidase (CD73): crystal form II (open) in complex with PSB11552 [Homo sapiens],6TVE_P Unliganded human CD73 (5'-nucleotidase) in the open state [Homo sapiens],6TVG_A Human CD73 (ecto 5'-nucleotidase) in complex with AMPCP in the open state [Homo sapiens],7BBJ_A Chain A, 5'-nucleotidase [Homo sapiens],7BBJ_B Chain B, 5'-nucleotidase [Homo sapiens],7P9N_A Chain A, 5'-nucleotidase [Homo sapiens],7P9R_A Chain A, 5'-nucleotidase [Homo sapiens],7P9T_A Chain A, 5'-nucleotidase [Homo sapiens],7PA4_A Chain A, 5'-nucleotidase [Homo sapiens],7PB5_A Chain A, 5'-nucleotidase [Homo sapiens],7PBA_A Chain A, 5'-nucleotidase [Homo sapiens],7PBB_A Chain A, 5'-nucleotidase [Homo sapiens],7PBY_A Chain A, 5'-nucleotidase [Homo sapiens],7PCP_A Chain A, 5'-nucleotidase [Homo sapiens],7PD9_A Chain A, 5'-nucleotidase [Homo sapiens] |
| 4H2B_A | 1.73e-49 | 35 | 508 | 26 | 544 | Humanecto-5'-nucleotidase (CD73): crystal form II (open) in complex with Baicalin [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A9BJC1 | 5.65e-65 | 34 | 495 | 22 | 482 | Mannosylglucosyl-3-phosphoglycerate phosphatase OS=Petrotoga mobilis (strain DSM 10674 / SJ95) OX=403833 GN=mggB PE=1 SV=1 |
| O34313 | 9.30e-65 | 35 | 508 | 668 | 1174 | Trifunctional nucleotide phosphoesterase protein YfkN OS=Bacillus subtilis (strain 168) OX=224308 GN=yfkN PE=1 SV=1 |
| P29240 | 8.75e-53 | 21 | 515 | 17 | 553 | 5'-nucleotidase OS=Diplobatis ommata OX=1870830 PE=2 SV=1 |
| B6EWW8 | 1.45e-52 | 25 | 508 | 33 | 561 | Snake venom 5'-nucleotidase OS=Gloydius brevicaudus OX=259325 PE=2 SV=1 |
| F8S0Z7 | 3.76e-52 | 25 | 508 | 33 | 561 | Snake venom 5'-nucleotidase OS=Crotalus adamanteus OX=8729 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
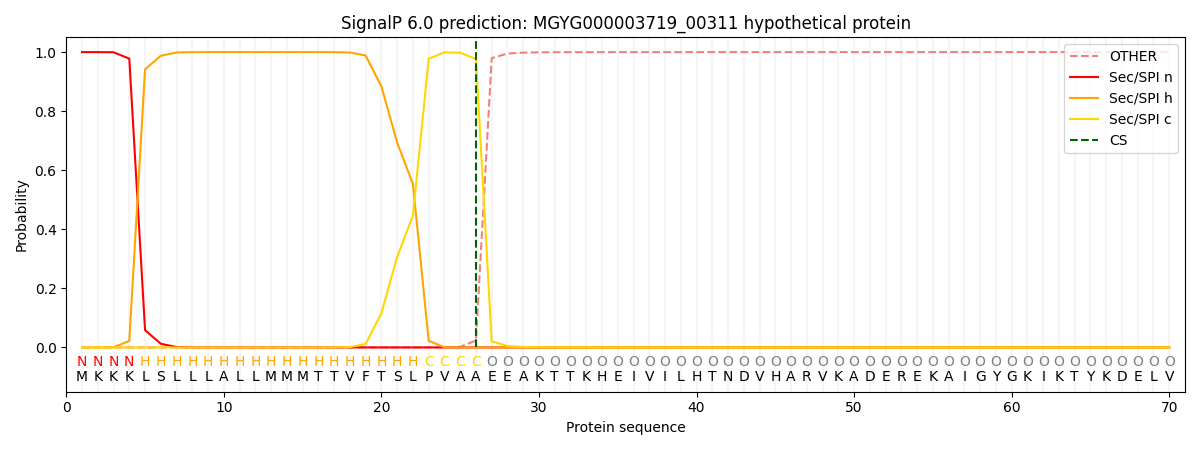
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000278 | 0.998978 | 0.000228 | 0.000172 | 0.000158 | 0.000144 |