You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003957_03428
You are here: Home > Sequence: MGYG000003957_03428
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
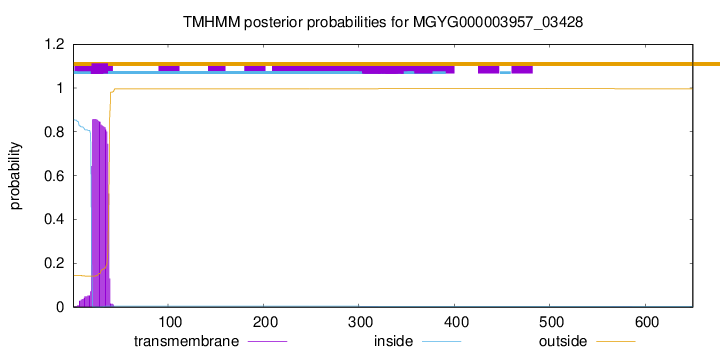
TMHMM annotations
Basic Information help
| Species | Parabacteroides sp014287585 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides sp014287585 | |||||||||||
| CAZyme ID | MGYG000003957_03428 | |||||||||||
| CAZy Family | CE17 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8977; End: 10929 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE17 | 305 | 471 | 2e-16 | 0.9878787878787879 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam13472 | Lipase_GDSL_2 | 3.64e-13 | 305 | 472 | 1 | 175 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| cd00229 | SGNH_hydrolase | 3.89e-12 | 303 | 478 | 1 | 184 | SGNH_hydrolase, or GDSL_hydrolase, is a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxlic acid. |
| cd01834 | SGNH_hydrolase_like_2 | 2.34e-06 | 302 | 373 | 3 | 73 | SGNH_hydrolase subfamily. SGNH hydrolases are a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| pfam00561 | Abhydrolase_1 | 4.32e-06 | 76 | 170 | 10 | 110 | alpha/beta hydrolase fold. This catalytic domain is found in a very wide range of enzymes. |
| COG1506 | DAP2 | 1.86e-05 | 79 | 272 | 412 | 616 | Dipeptidyl aminopeptidase/acylaminoacyl peptidase [Amino acid transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AEE54090.1 | 4.65e-153 | 38 | 648 | 394 | 1015 |
| QEC52777.1 | 1.68e-82 | 286 | 648 | 823 | 1251 |
| BAV07942.1 | 2.53e-70 | 283 | 649 | 31 | 394 |
| AHF91789.1 | 2.53e-28 | 285 | 608 | 40 | 377 |
| AXT62271.1 | 4.87e-22 | 284 | 647 | 35 | 394 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6HH9_A | 3.81e-15 | 303 | 620 | 35 | 354 | Crystalstructure of a two-domain esterase (CEX) active on acetylated mannans co-crystallized with mannopentaose [Roseburia intestinalis L1-82],6HH9_B Crystal structure of a two-domain esterase (CEX) active on acetylated mannans co-crystallized with mannopentaose [Roseburia intestinalis L1-82],6HH9_C Crystal structure of a two-domain esterase (CEX) active on acetylated mannans co-crystallized with mannopentaose [Roseburia intestinalis L1-82],6HH9_D Crystal structure of a two-domain esterase (CEX) active on acetylated mannans co-crystallized with mannopentaose [Roseburia intestinalis L1-82] |
| 6HFZ_A | 1.21e-14 | 303 | 620 | 35 | 354 | Crystalstructure of a two-domain esterase (CEX) active on acetylated mannans [Roseburia intestinalis L1-82],6HFZ_B Crystal structure of a two-domain esterase (CEX) active on acetylated mannans [Roseburia intestinalis L1-82],6HFZ_C Crystal structure of a two-domain esterase (CEX) active on acetylated mannans [Roseburia intestinalis L1-82],6HFZ_D Crystal structure of a two-domain esterase (CEX) active on acetylated mannans [Roseburia intestinalis L1-82] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
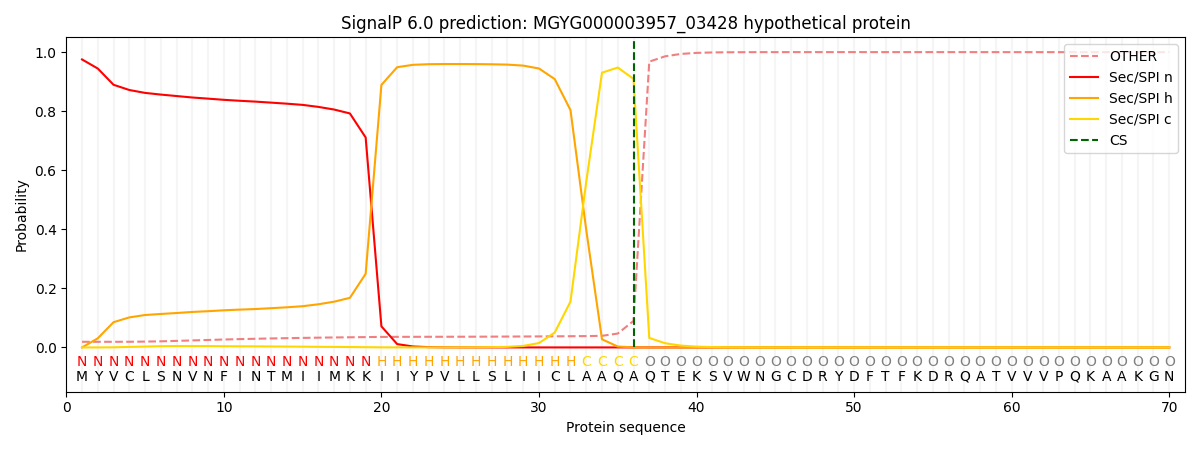
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.020583 | 0.971680 | 0.006923 | 0.000320 | 0.000225 | 0.000235 |