You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004223_01596
You are here: Home > Sequence: MGYG000004223_01596
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
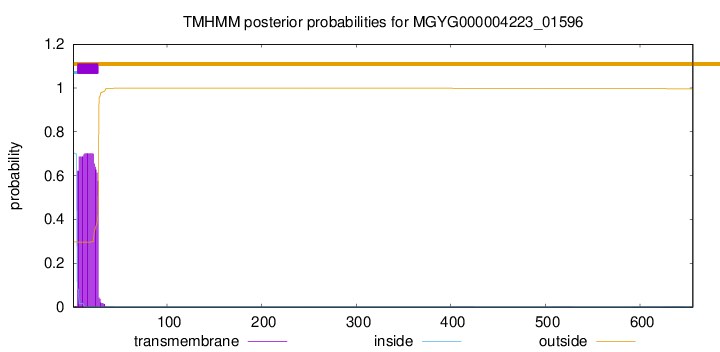
TMHMM annotations
Basic Information help
| Species | UMGS1883 sp900763305 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; UMGS1883; UMGS1883; UMGS1883; UMGS1883 sp900763305 | |||||||||||
| CAZyme ID | MGYG000004223_01596 | |||||||||||
| CAZy Family | GH26 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 47458; End: 49428 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH26 | 342 | 490 | 6.3e-19 | 0.44554455445544555 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam07833 | Cu_amine_oxidN1 | 3.53e-28 | 44 | 132 | 1 | 89 | Copper amine oxidase N-terminal domain. Copper amine oxidases catalyze the oxidative deamination of primary amines to the corresponding aldehydes, while reducing molecular oxygen to hydrogen peroxide. These enzymes are dimers of identical subunits, each comprising four domains. The N-terminal domain, which is absent in some amine oxidases, consists of a five-stranded antiparallel beta sheet twisted around an alpha helix. The D1 domains from the two subunits comprise the 'stalk' of the mushroom-shaped dimer, and interact with each other but do not pack tightly against each other. |
| pfam07833 | Cu_amine_oxidN1 | 6.10e-08 | 19 | 74 | 41 | 93 | Copper amine oxidase N-terminal domain. Copper amine oxidases catalyze the oxidative deamination of primary amines to the corresponding aldehydes, while reducing molecular oxygen to hydrogen peroxide. These enzymes are dimers of identical subunits, each comprising four domains. The N-terminal domain, which is absent in some amine oxidases, consists of a five-stranded antiparallel beta sheet twisted around an alpha helix. The D1 domains from the two subunits comprise the 'stalk' of the mushroom-shaped dimer, and interact with each other but do not pack tightly against each other. |
| COG4124 | ManB2 | 1.25e-06 | 271 | 513 | 77 | 336 | Beta-mannanase [Carbohydrate transport and metabolism]. |
| PRK14696 | tynA | 0.002 | 49 | 112 | 1 | 64 | primary-amine oxidase. |
| pfam02156 | Glyco_hydro_26 | 0.003 | 363 | 449 | 143 | 232 | Glycosyl hydrolase family 26. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QSX05302.1 | 7.53e-179 | 19 | 655 | 26 | 665 |
| AZN43604.1 | 6.99e-102 | 157 | 649 | 31 | 530 |
| QNK58256.1 | 2.44e-96 | 152 | 656 | 31 | 538 |
| QHT63759.1 | 1.38e-95 | 158 | 656 | 4 | 507 |
| AMY96565.1 | 3.04e-94 | 199 | 656 | 1 | 461 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
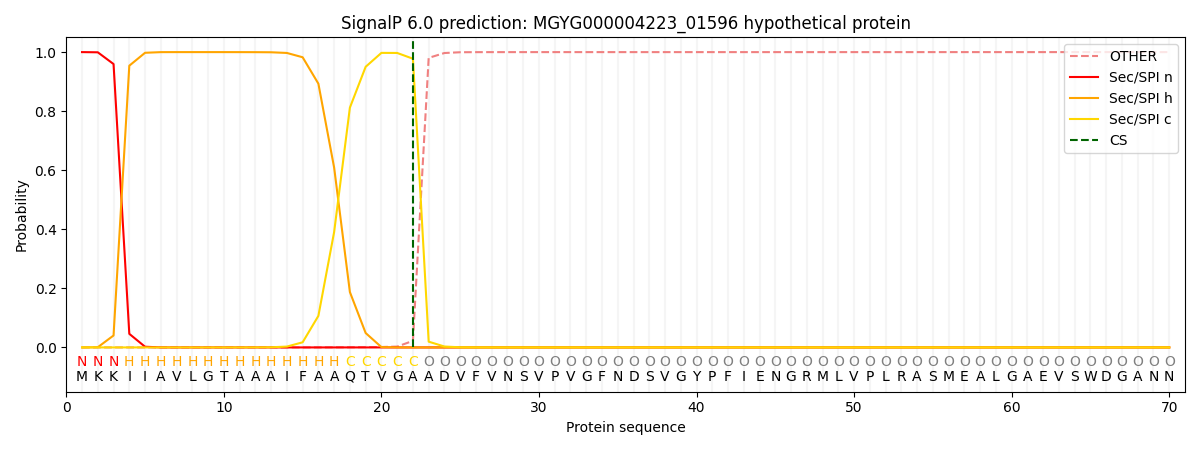
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000228 | 0.999103 | 0.000164 | 0.000168 | 0.000160 | 0.000146 |