You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004253_00485
You are here: Home > Sequence: MGYG000004253_00485
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
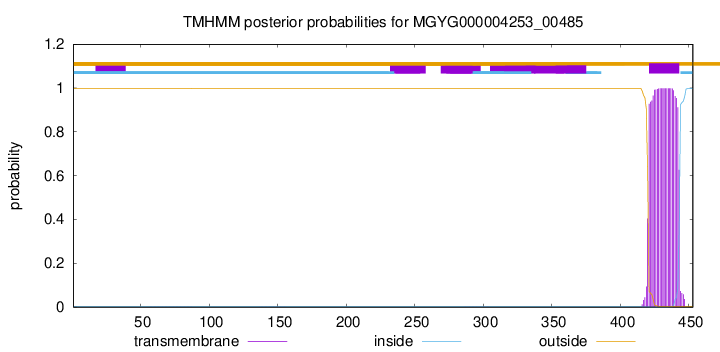
TMHMM annotations
Basic Information help
| Species | Desulfovibrio piger_A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Desulfobacterota; Desulfovibrionia; Desulfovibrionales; Desulfovibrionaceae; Desulfovibrio; Desulfovibrio piger_A | |||||||||||
| CAZyme ID | MGYG000004253_00485 | |||||||||||
| CAZy Family | GT4 | |||||||||||
| CAZyme Description | D-inositol-3-phosphate glycosyltransferase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 12958; End: 14319 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd03825 | GT4_WcaC-like | 1.18e-94 | 32 | 371 | 31 | 364 | putative colanic acid biosynthesis glycosyl transferase WcaC and similar proteins. This family is most closely related to the GT4 family of glycosyltransferases. Escherichia coli WcaC has been predicted to function in colanic acid biosynthesis. WcfI in Bacteroides fragilis has been shown to be involved in the capsular polysaccharide biosynthesis. |
| cd03801 | GT4_PimA-like | 2.13e-27 | 44 | 368 | 72 | 365 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| COG0438 | RfaB | 7.55e-26 | 38 | 374 | 67 | 380 | Glycosyltransferase involved in cell wall bisynthesis [Cell wall/membrane/envelope biogenesis]. |
| cd03823 | GT4_ExpE7-like | 2.98e-17 | 20 | 365 | 68 | 356 | glycosyltransferase ExpE7 and similar proteins. This family is most closely related to the GT4 family of glycosyltransferases. ExpE7 in Sinorhizobium meliloti has been shown to be involved in the biosynthesis of galactoglucans (exopolysaccharide II). |
| cd03798 | GT4_WlbH-like | 6.53e-17 | 136 | 371 | 145 | 376 | Bordetella parapertussis WlbH and similar proteins. This family is most closely related to the GT4 family of glycosyltransferases. Staphylococcus aureus CapJ may be involved in capsule polysaccharide biosynthesis. WlbH in Bordetella parapertussis has been shown to be required for the biosynthesis of a trisaccharide that, when attached to the B. pertussis lipopolysaccharide (LPS) core (band B), generates band A LPS. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| SFV73268.1 | 0.0 | 1 | 453 | 32 | 484 |
| QMV41668.1 | 3.57e-86 | 33 | 375 | 71 | 412 |
| QHG18542.1 | 1.45e-76 | 48 | 375 | 82 | 407 |
| AFZ46690.1 | 2.12e-75 | 24 | 373 | 58 | 405 |
| QLE51298.1 | 2.25e-75 | 48 | 375 | 82 | 407 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3C4Q_A | 1.04e-15 | 173 | 372 | 196 | 406 | Structureof the retaining glycosyltransferase MshA : The first step in mycothiol biosynthesis. Organism : Corynebacterium glutamicum- Complex with UDP [Corynebacterium glutamicum],3C4Q_B Structure of the retaining glycosyltransferase MshA : The first step in mycothiol biosynthesis. Organism : Corynebacterium glutamicum- Complex with UDP [Corynebacterium glutamicum],3C4V_A Structure of the retaining glycosyltransferase MshA:The first step in mycothiol biosynthesis. Organism: Corynebacterium glutamicum : Complex with UDP and 1L-INS-1-P. [Corynebacterium glutamicum],3C4V_B Structure of the retaining glycosyltransferase MshA:The first step in mycothiol biosynthesis. Organism: Corynebacterium glutamicum : Complex with UDP and 1L-INS-1-P. [Corynebacterium glutamicum] |
| 3C48_A | 1.10e-15 | 173 | 372 | 216 | 426 | Structureof the retaining glycosyltransferase MshA: The first step in mycothiol biosynthesis. Organism: Corynebacterium glutamicum- APO (OPEN) structure. [Corynebacterium glutamicum],3C48_B Structure of the retaining glycosyltransferase MshA: The first step in mycothiol biosynthesis. Organism: Corynebacterium glutamicum- APO (OPEN) structure. [Corynebacterium glutamicum] |
| 2JJM_A | 5.16e-13 | 257 | 372 | 271 | 386 | CrystalStructure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_B Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_C Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_D Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_E Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_F Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_G Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_H Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_I Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_J Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_K Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_L Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames] |
| 3MBO_A | 5.65e-13 | 257 | 372 | 291 | 406 | CrystalStructure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_B Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_C Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_D Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_E Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_F Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_G Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_H Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis] |
| 5D00_A | 1.14e-12 | 267 | 373 | 271 | 377 | Crystalstructure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate and UMP [Bacillus subtilis subsp. subtilis str. 168],5D00_B Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate and UMP [Bacillus subtilis subsp. subtilis str. 168],5D01_A Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate [Bacillus subtilis subsp. subtilis str. 168],5D01_B Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate [Bacillus subtilis subsp. subtilis str. 168] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A1SP12 | 1.99e-17 | 173 | 347 | 235 | 427 | D-inositol 3-phosphate glycosyltransferase OS=Nocardioides sp. (strain ATCC BAA-499 / JS614) OX=196162 GN=mshA PE=3 SV=1 |
| D3Q051 | 5.94e-17 | 171 | 387 | 216 | 442 | D-inositol 3-phosphate glycosyltransferase OS=Stackebrandtia nassauensis (strain DSM 44728 / CIP 108903 / NRRL B-16338 / NBRC 102104 / LLR-40K-21) OX=446470 GN=mshA PE=3 SV=1 |
| A0LQY9 | 8.15e-17 | 170 | 383 | 220 | 443 | D-inositol 3-phosphate glycosyltransferase OS=Acidothermus cellulolyticus (strain ATCC 43068 / DSM 8971 / 11B) OX=351607 GN=mshA PE=3 SV=1 |
| C6WPK3 | 1.23e-16 | 167 | 370 | 192 | 406 | D-inositol 3-phosphate glycosyltransferase OS=Actinosynnema mirum (strain ATCC 29888 / DSM 43827 / JCM 3225 / NBRC 14064 / NCIMB 13271 / NRRL B-12336 / IMRU 3971 / 101) OX=446462 GN=mshA PE=3 SV=1 |
| C3PK12 | 2.27e-16 | 56 | 372 | 104 | 406 | D-inositol 3-phosphate glycosyltransferase OS=Corynebacterium aurimucosum (strain ATCC 700975 / DSM 44827 / CIP 107346 / CN-1) OX=548476 GN=mshA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
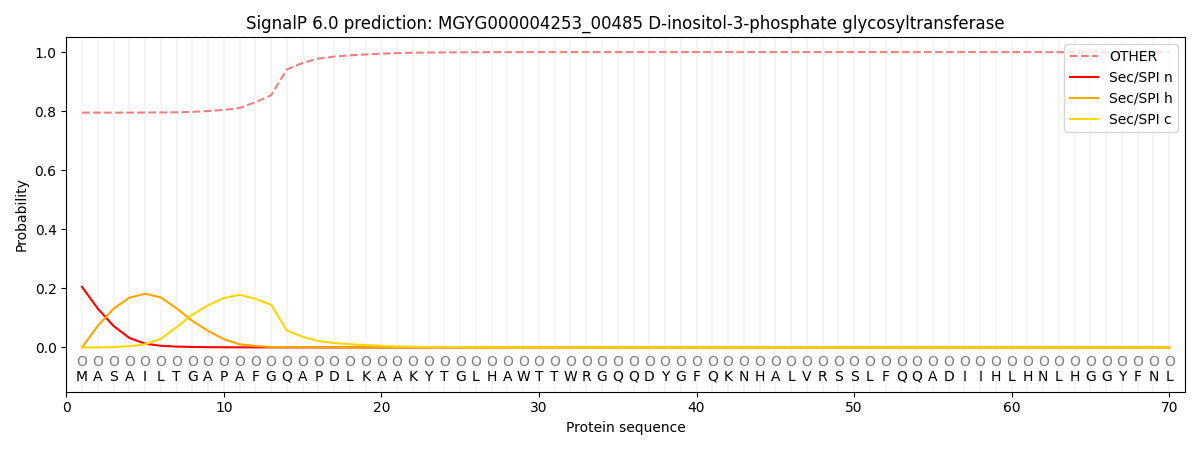
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.805510 | 0.193762 | 0.000255 | 0.000215 | 0.000121 | 0.000167 |