You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004282_00821
You are here: Home > Sequence: MGYG000004282_00821
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Ruminococcus_D sp000434695 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus_D; Ruminococcus_D sp000434695 | |||||||||||
| CAZyme ID | MGYG000004282_00821 | |||||||||||
| CAZy Family | GH74 | |||||||||||
| CAZyme Description | Xyloglucanase Xgh74A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 32693; End: 34978 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH74 | 108 | 215 | 6.4e-20 | 0.4678111587982833 |
| GH74 | 598 | 741 | 9e-16 | 0.4206008583690987 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd15482 | Sialidase_non-viral | 3.15e-06 | 194 | 378 | 115 | 297 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| pfam15902 | Sortilin-Vps10 | 3.26e-05 | 573 | 723 | 2 | 167 | Sortilin, neurotensin receptor 3,. Sortilin, also known in mammals as neurotensin receptor-3, is the archetypical member of a Vps10-domain (Vps10-D) that binds neurotrophic factors and neuropeptides. This domain constitutes the entire luminal part of Sortilin and is activated in the trans-Golgi network by enzymatic propeptide cleavage. The structure of the domain has been determined as a ten-bladed propeller, with up to 9 BNR or beta-hairpin turns in it. The mature receptor binds various ligands, including its own propeptide (Sort-pro), neurotensin, the pro-forms of nerve growth factor-beta (NGF)6 and brain-derived neurotrophic factor (BDNF)7, lipoprotein lipase (LpL), apo lipoprotein AV14 and the receptor-associated protein (RAP)1. |
| smart00602 | VPS10 | 3.04e-04 | 76 | 204 | 8 | 112 | VPS10 domain. |
| pfam15902 | Sortilin-Vps10 | 6.61e-04 | 525 | 630 | 2 | 115 | Sortilin, neurotensin receptor 3,. Sortilin, also known in mammals as neurotensin receptor-3, is the archetypical member of a Vps10-domain (Vps10-D) that binds neurotrophic factors and neuropeptides. This domain constitutes the entire luminal part of Sortilin and is activated in the trans-Golgi network by enzymatic propeptide cleavage. The structure of the domain has been determined as a ten-bladed propeller, with up to 9 BNR or beta-hairpin turns in it. The mature receptor binds various ligands, including its own propeptide (Sort-pro), neurotensin, the pro-forms of nerve growth factor-beta (NGF)6 and brain-derived neurotrophic factor (BDNF)7, lipoprotein lipase (LpL), apo lipoprotein AV14 and the receptor-associated protein (RAP)1. |
| pfam15902 | Sortilin-Vps10 | 7.79e-04 | 525 | 629 | 46 | 166 | Sortilin, neurotensin receptor 3,. Sortilin, also known in mammals as neurotensin receptor-3, is the archetypical member of a Vps10-domain (Vps10-D) that binds neurotrophic factors and neuropeptides. This domain constitutes the entire luminal part of Sortilin and is activated in the trans-Golgi network by enzymatic propeptide cleavage. The structure of the domain has been determined as a ten-bladed propeller, with up to 9 BNR or beta-hairpin turns in it. The mature receptor binds various ligands, including its own propeptide (Sort-pro), neurotensin, the pro-forms of nerve growth factor-beta (NGF)6 and brain-derived neurotrophic factor (BDNF)7, lipoprotein lipase (LpL), apo lipoprotein AV14 and the receptor-associated protein (RAP)1. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CCO05647.1 | 0.0 | 1 | 760 | 1 | 762 |
| ADU20593.1 | 0.0 | 3 | 759 | 4 | 768 |
| CBL16561.1 | 1.41e-192 | 54 | 758 | 34 | 725 |
| AQR94478.1 | 6.61e-174 | 1 | 761 | 1 | 786 |
| ANY71781.1 | 2.56e-171 | 51 | 761 | 42 | 778 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2CN3_A | 1.14e-155 | 54 | 761 | 11 | 734 | ChainA, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus],2CN3_B Chain B, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus] |
| 6MGL_A | 6.69e-154 | 60 | 754 | 13 | 742 | Crystalstructure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, D60A mutant in complex with XXLG and XGXXLG xyloglucan [Paenibacillus odorifer] |
| 2CN2_A | 1.40e-153 | 54 | 761 | 11 | 734 | ChainA, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus],2CN2_B Chain B, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus],2CN2_C Chain C, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus],2CN2_D Chain D, BETA-1,4-XYLOGLUCAN HYDROLASE [Acetivibrio thermocellus] |
| 4LGN_A | 1.05e-149 | 54 | 758 | 7 | 735 | Thestructure of Acidothermus cellulolyticus family 74 glycoside hydrolase [Acidothermus cellulolyticus 11B] |
| 6MGJ_A | 1.97e-149 | 60 | 754 | 13 | 742 | Crystalstructure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_B Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_C Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_D Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_E Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_F Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_G Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGJ_H Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, apoenzyme [Paenibacillus odorifer],6MGK_A Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, in complex with XLX xyloglucan [Paenibacillus odorifer],6MGK_B Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, in complex with XLX xyloglucan [Paenibacillus odorifer],6MGK_C Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, in complex with XLX xyloglucan [Paenibacillus odorifer],6MGK_D Crystal structure of the catalytic domain from GH74 enzyme PoGH74 from Paenibacillus odorifer, in complex with XLX xyloglucan [Paenibacillus odorifer] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A3DFA0 | 2.09e-155 | 54 | 761 | 38 | 761 | Xyloglucanase Xgh74A OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=xghA PE=3 SV=1 |
| Q70DK5 | 8.21e-155 | 54 | 761 | 38 | 761 | Xyloglucanase Xgh74A OS=Acetivibrio thermocellus OX=1515 GN=xghA PE=1 SV=1 |
| Q3MUH7 | 5.31e-153 | 54 | 754 | 38 | 762 | Xyloglucanase OS=Paenibacillus sp. OX=58172 GN=xeg74 PE=1 SV=1 |
| A1DAU0 | 9.75e-118 | 47 | 758 | 20 | 798 | Probable oligoxyloglucan-reducing end-specific xyloglucanase OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=xgcA PE=3 SV=1 |
| Q5BD38 | 6.16e-112 | 47 | 760 | 28 | 810 | Oligoxyloglucan-reducing end-specific xyloglucanase OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=xgcA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
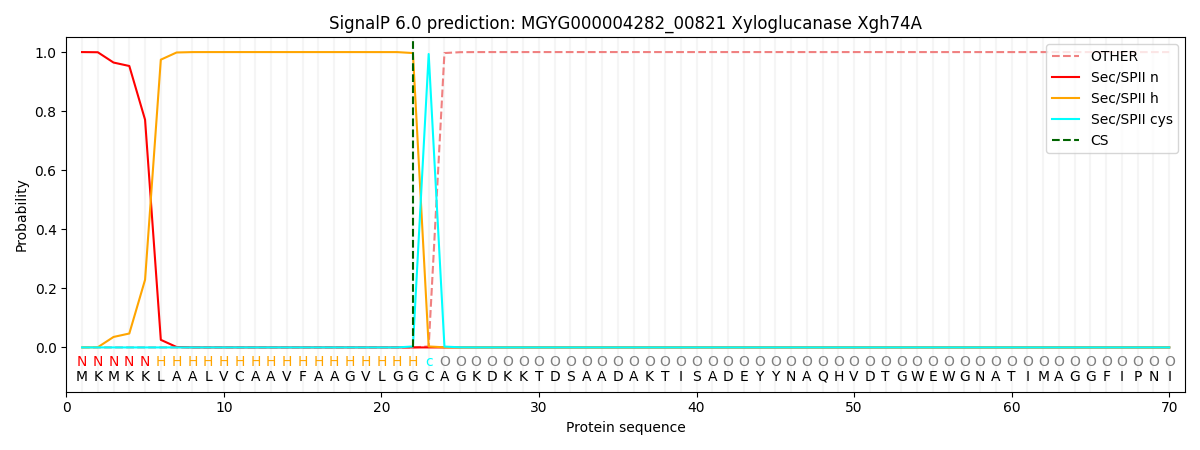
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000000 | 1.000073 | 0.000000 | 0.000000 | 0.000000 |
