You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004304_00266
You are here: Home > Sequence: MGYG000004304_00266
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; UBA1394; | |||||||||||
| CAZyme ID | MGYG000004304_00266 | |||||||||||
| CAZy Family | GH24 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 5839; End: 7758 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH24 | 32 | 173 | 2.4e-29 | 0.9927007299270073 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd00737 | lyz_endolysin_autolysin | 9.71e-35 | 36 | 178 | 1 | 136 | endolysin and autolysin. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| COG3772 | RrrD | 1.94e-20 | 30 | 180 | 5 | 150 | Phage-related lysozyme (muramidase), GH24 family [Cell wall/membrane/envelope biogenesis]. |
| cd16901 | lyz_P1 | 1.41e-15 | 31 | 176 | 1 | 138 | P1 lysozyme Lyz-like proteins. Enterobacteria phage P1 lysozyme Lyz is secreted to the Escherichia coli periplasm where it is membrane bound and inactive. Activation involves the release from the membrane, an intramolecular thiol-disulfide isomerization and extensive structural rearrangement of the N-terminal region. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| cd14256 | Dockerin_I | 2.73e-09 | 580 | 636 | 1 | 56 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| cd16900 | endolysin_R21-like | 9.51e-08 | 39 | 178 | 11 | 142 | endolysin R21-like proteins. Unlike T4 E phage lysozyme, the endolysin R21 from Enterobacteria phage P21 has an N-terminal SAR (signal-arrest-release) domain that anchors the endolysin to the membrane in an inactive form, which act to prevent premature lysis of the infected bacterium. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ACR76485.1 | 3.30e-35 | 25 | 240 | 71 | 288 |
| ADQ05619.1 | 1.83e-27 | 345 | 575 | 249 | 498 |
| ADD02972.1 | 1.18e-24 | 345 | 632 | 230 | 520 |
| QUW24060.1 | 1.96e-24 | 286 | 572 | 215 | 518 |
| BAC87940.1 | 2.40e-24 | 288 | 576 | 248 | 555 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6ET6_A | 1.67e-13 | 33 | 178 | 53 | 194 | ChainA, Lysozyme [Acinetobacter baumannii] |
| 6H9D_A | 2.48e-06 | 31 | 179 | 5 | 148 | ChainA, Lysozyme [Asticcacaulis excentricus],6H9D_B Chain B, Lysozyme [Asticcacaulis excentricus],6H9D_C Chain C, Lysozyme [Asticcacaulis excentricus] |
| 7M5I_A | 3.02e-06 | 25 | 178 | 2 | 154 | ChainA, Endolysin [Escherichia coli O157 typing phage 15],7M5I_B Chain B, Endolysin [Escherichia coli O157 typing phage 15] |
| 5LXV_B | 7.84e-06 | 581 | 629 | 26 | 70 | Crystalstructure of Ruminococcus flavefaciens scaffoldin C cohesin in complex with a dockerin from an uncharacterized CBM-containing protein [Ruminococcus flavefaciens FD-1],5LXV_D Crystal structure of Ruminococcus flavefaciens scaffoldin C cohesin in complex with a dockerin from an uncharacterized CBM-containing protein [Ruminococcus flavefaciens FD-1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P07540 | 1.32e-13 | 30 | 179 | 1 | 144 | Endolysin OS=Bacillus phage PZA OX=10757 GN=15 PE=3 SV=1 |
| P11187 | 1.32e-13 | 30 | 179 | 1 | 144 | Endolysin OS=Bacillus phage phi29 OX=10756 GN=15 PE=1 SV=1 |
| Q37896 | 2.10e-12 | 30 | 217 | 1 | 184 | Endolysin OS=Bacillus phage B103 OX=10778 GN=15 PE=3 SV=1 |
| P62692 | 8.04e-11 | 30 | 177 | 1 | 136 | Endolysin OS=Lactococcus phage c2 OX=31537 GN=L3 PE=3 SV=1 |
| P62693 | 8.04e-11 | 30 | 177 | 1 | 136 | Endolysin OS=Lactococcus phage phivML3 OX=10746 GN=L3 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
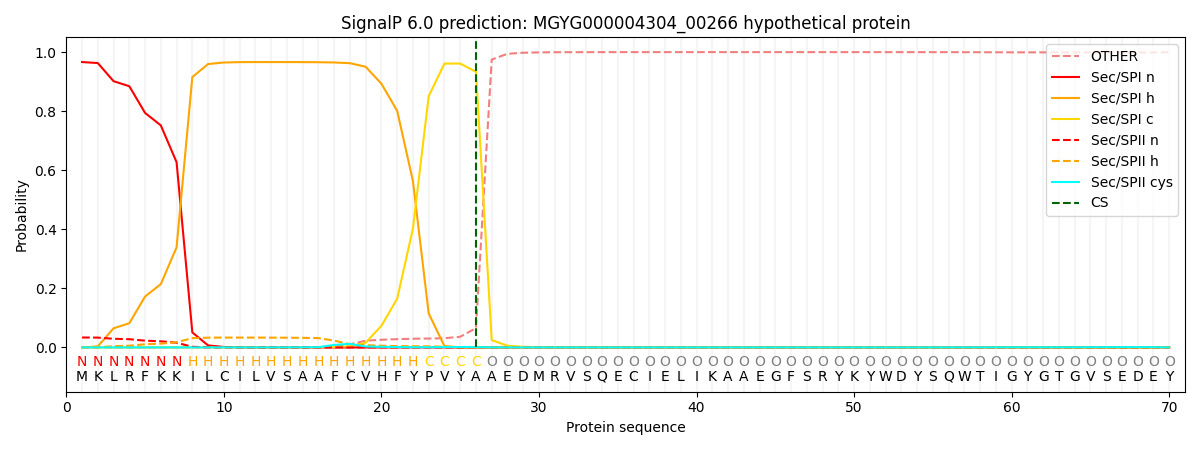
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001061 | 0.957461 | 0.040468 | 0.000316 | 0.000333 | 0.000316 |
