You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004382_01260
You are here: Home > Sequence: MGYG000004382_01260
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
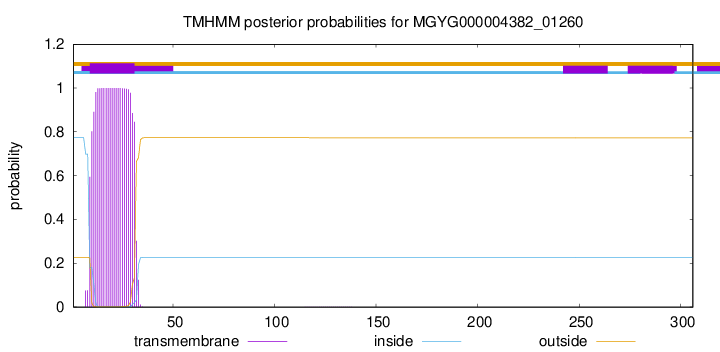
TMHMM annotations
Basic Information help
| Species | UBA2821 sp902763335 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; UBA2821; UBA2821 sp902763335 | |||||||||||
| CAZyme ID | MGYG000004382_01260 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 45560; End: 46480 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 100 | 219 | 3.4e-22 | 0.8615384615384616 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10944 | CE4_SmPgdA_like | 3.49e-78 | 103 | 291 | 1 | 189 | Catalytic NodB homology domain of Streptococcus mutans polysaccharide deacetylase PgdA, Bacillus subtilis YheN, and similar proteins. This family is represented by a putative polysaccharide deacetylase PgdA from the oral pathogen Streptococcus mutans (SmPgdA) and Bacillus subtilis YheN (BsYheN), which are members of the carbohydrate esterase 4 (CE4) superfamily. SmPgdA is an extracellular metal-dependent polysaccharide deacetylase with a typical CE4 fold, with metal bound to a His-His-Asp triad. It possesses de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. SmPgdA plays a role in tuning cell surface properties and in interactions with (salivary) agglutinin, an essential component of the innate immune system, most likely through deacetylation of an as-yet-unidentified polysaccharide. SmPgdA shows significant homology to the catalytic domains of peptidoglycan deacetylases from Streptococcus pneumoniae (SpPgdA) and Listeria monocytogenes (LmPgdA), both of which are involved in the bacterial defense mechanism against human mucosal lysozyme. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. The biological function of BsYheN is still unknown. This family also includes many uncharacterized polysaccharide deacetylases mainly found in bacteria. |
| cd10917 | CE4_NodB_like_6s_7s | 9.90e-40 | 103 | 283 | 1 | 171 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| cd10951 | CE4_ClCDA_like | 1.70e-30 | 107 | 289 | 12 | 194 | Catalytic NodB homology domain of Colletotrichum lindemuthianum chitin deacetylase and similar proteins. This family is represented by the chitin deacetylase (endo-chitin de-N-acetylase, ClCDA, EC 3.5.1.41) from Colletotrichum lindemuthianum (also known as Glomerella lindemuthiana), which is a member of the carbohydrate esterase 4 (CE4) superfamily. ClCDA catalyzes the hydrolysis of N-acetamido groups of N-acetyl-D-glucosamine residues in chitin, converting it to chitosan in fungal cell walls. It consists of a single catalytic domain similar to the deformed (alpha/beta)8 barrel fold adopted by other CE4 esterases, which encompasses a mononuclear metalloenzyme employing a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine), to carry out acid/base catalysis. It possesses a highly conserved substrate-binding groove, with subtle alterations that influence substrate specificity and subsite affinity. Unlike its bacterial homologs, ClCDA contains two intramolecular disulfide bonds that may add stability to this secreted protein. The family also includes many uncharacterized deacetylases and hypothetical proteins mainly from eukaryotes, which show high sequence similarity to ClCDA. |
| COG0726 | CDA1 | 2.75e-30 | 97 | 297 | 59 | 258 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd10959 | CE4_NodB_like_3 | 6.80e-30 | 103 | 289 | 1 | 184 | Catalytic NodB homology domain of uncharacterized bacterial polysaccharide deacetylases. This family includes many uncharacterized bacterial polysaccharide deacetylases. Although their biological function still remains unknown, members in this family show high sequence homology to the catalytic NodB homology domain of Streptococcus pneumoniae polysaccharide deacetylase PgdA (SpPgdA), which is an extracellular metal-dependent polysaccharide deacetylase with de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. Like SpPgdA, this family is a member of the carbohydrate esterase 4 (CE4) superfamily. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QOY61113.1 | 2.89e-48 | 102 | 295 | 99 | 296 |
| QIB68221.1 | 2.85e-47 | 10 | 305 | 12 | 302 |
| QAT41967.1 | 2.85e-47 | 103 | 305 | 98 | 302 |
| ARQ78351.1 | 3.52e-47 | 101 | 295 | 88 | 287 |
| QDX33051.1 | 3.52e-47 | 101 | 295 | 88 | 287 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5JMU_A | 6.78e-34 | 96 | 289 | 13 | 210 | ChainA, Peptidoglycan N-acetylglucosamine deacetylase [[Eubacterium] rectale ATCC 33656] |
| 2W3Z_A | 7.49e-27 | 102 | 292 | 106 | 310 | Structureof a Streptococcus mutans CE4 esterase [Streptococcus mutans UA159] |
| 7FBW_A | 3.81e-20 | 103 | 297 | 117 | 301 | ChainA, Predicted xylanase/chitin deacetylase [Caldanaerobacter subterraneus subsp. tengcongensis MB4] |
| 5NC6_A | 1.62e-17 | 103 | 290 | 1 | 187 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (E)-N-hydroxy-3-(naphthalen-1-yl)prop-2-enamide [Bacillus cereus] |
| 5N1J_A | 1.66e-17 | 103 | 290 | 2 | 188 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1P_A Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O07596 | 1.51e-19 | 102 | 296 | 84 | 277 | Putative polysaccharide deacetylase YheN OS=Bacillus subtilis (strain 168) OX=224308 GN=yheN PE=3 SV=1 |
| Q81EJ6 | 1.48e-16 | 84 | 290 | 54 | 255 | Peptidoglycan-N-acetylglucosamine deacetylase BC_1974 OS=Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG 7414 / JCM 2152 / NBRC 15305 / NCIMB 9373 / NCTC 2599 / NRRL B-3711) OX=226900 GN=BC_1974 PE=1 SV=1 |
| Q04729 | 4.28e-15 | 99 | 294 | 63 | 252 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
| O34928 | 2.74e-14 | 102 | 294 | 65 | 251 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaA PE=1 SV=1 |
| P83513 | 4.82e-14 | 100 | 298 | 399 | 586 | Bifunctional xylanase/deacetylase OS=Pseudobutyrivibrio xylanivorans OX=185007 GN=xyn11A PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
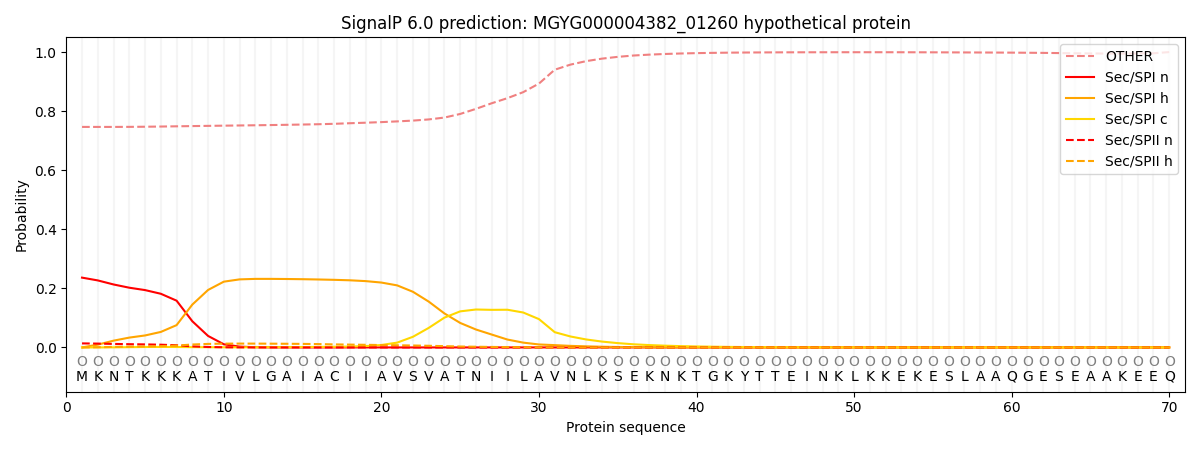
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.760010 | 0.218837 | 0.014910 | 0.001028 | 0.000542 | 0.004666 |