You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004570_00655
You are here: Home > Sequence: MGYG000004570_00655
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
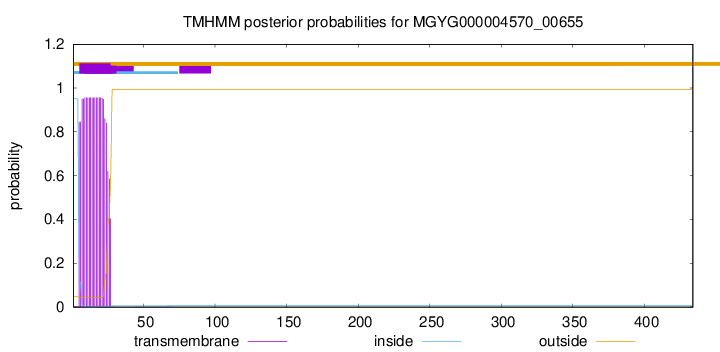
TMHMM annotations
Basic Information help
| Species | Treponema_D berlinense | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Spirochaetota; Spirochaetia; Treponematales; Treponemataceae; Treponema_D; Treponema_D berlinense | |||||||||||
| CAZyme ID | MGYG000004570_00655 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | Pectate lyase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 84211; End: 85515 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 164 | 367 | 1.1e-65 | 0.971830985915493 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00656 | Amb_all | 5.80e-50 | 174 | 367 | 17 | 186 | Amb_all domain. |
| COG3866 | PelB | 7.76e-48 | 29 | 430 | 18 | 344 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam00544 | Pec_lyase_C | 1.32e-36 | 174 | 367 | 35 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AEJ18226.1 | 9.40e-118 | 16 | 434 | 45 | 475 |
| AEE17959.1 | 8.89e-81 | 8 | 428 | 17 | 442 |
| QPH91275.1 | 3.39e-67 | 11 | 434 | 5 | 416 |
| QPI07285.1 | 2.84e-65 | 1 | 434 | 1 | 416 |
| QPH93180.1 | 7.87e-65 | 11 | 434 | 5 | 416 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1VBL_A | 1.05e-48 | 31 | 428 | 12 | 413 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
| 1BN8_A | 4.60e-45 | 1 | 426 | 1 | 417 | BacillusSubtilis Pectate Lyase [Bacillus subtilis] |
| 5AMV_A | 1.12e-44 | 29 | 426 | 10 | 396 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
| 2BSP_A | 1.24e-44 | 1 | 426 | 1 | 417 | ChainA, PROTEIN (PECTATE LYASE) [Bacillus subtilis] |
| 2NZM_A | 8.15e-44 | 29 | 426 | 10 | 396 | ChainA, Pectate lyase [Bacillus subtilis],2O04_A Chain A, Pectate lyase [Bacillus subtilis],2O0V_A Chain A, Pectate lyase [Bacillus subtilis],2O0W_A Chain A, Pectate lyase [Bacillus subtilis],2O17_A Chain A, Pectate lyase [Bacillus subtilis],2O1D_A Chain A, Pectate lyase [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P39116 | 2.52e-44 | 1 | 426 | 1 | 417 | Pectate lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pel PE=1 SV=1 |
| P04960 | 4.71e-38 | 169 | 430 | 106 | 385 | Pectate lyase E OS=Dickeya chrysanthemi OX=556 GN=pelE PE=1 SV=1 |
| P18209 | 7.06e-36 | 169 | 344 | 112 | 282 | Pectate lyase D OS=Dickeya chrysanthemi OX=556 GN=pelD PE=3 SV=1 |
| P0C1A5 | 2.36e-35 | 169 | 430 | 124 | 404 | Pectate lyase E OS=Dickeya dadantii (strain 3937) OX=198628 GN=pelE PE=3 SV=2 |
| P0C1A4 | 8.63e-35 | 169 | 430 | 124 | 404 | Pectate lyase E OS=Dickeya chrysanthemi OX=556 GN=pelE PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000367 | 0.998719 | 0.000237 | 0.000236 | 0.000210 | 0.000197 |