You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004603_01319
You are here: Home > Sequence: MGYG000004603_01319
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
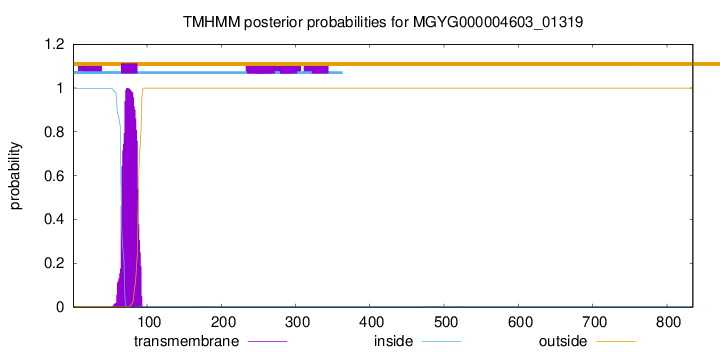
TMHMM annotations
Basic Information help
| Species | Streptococcus oralis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Lactobacillales; Streptococcaceae; Streptococcus; Streptococcus oralis | |||||||||||
| CAZyme ID | MGYG000004603_01319 | |||||||||||
| CAZy Family | GT51 | |||||||||||
| CAZyme Description | Monofunctional biosynthetic peptidoglycan transglycosylase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 99703; End: 102210 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT51 | 122 | 311 | 1.2e-49 | 0.9830508474576272 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG0744 | MrcB | 4.32e-161 | 63 | 773 | 11 | 660 | Membrane carboxypeptidase (penicillin-binding protein) [Cell wall/membrane/envelope biogenesis]. |
| COG5009 | MrcA | 5.89e-67 | 70 | 745 | 8 | 746 | Membrane carboxypeptidase/penicillin-binding protein [Cell wall/membrane/envelope biogenesis]. |
| COG4953 | PbpC | 2.61e-50 | 130 | 693 | 44 | 541 | Membrane carboxypeptidase/penicillin-binding protein PbpC [Cell wall/membrane/envelope biogenesis]. |
| pfam00912 | Transgly | 1.10e-49 | 121 | 312 | 1 | 177 | Transglycosylase. The penicillin-binding proteins are bifunctional proteins consisting of transglycosylase and transpeptidase in the N- and C-terminus respectively. The transglycosylase domain catalyzes the polymerization of murein glycan chains. |
| PRK11636 | mrcA | 4.12e-33 | 70 | 691 | 7 | 747 | penicillin-binding protein 1a; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QLL97670.1 | 0.0 | 1 | 835 | 1 | 835 |
| CBY99844.1 | 0.0 | 1 | 835 | 1 | 835 |
| QLL96513.1 | 0.0 | 1 | 835 | 1 | 839 |
| QRO07815.1 | 0.0 | 1 | 835 | 1 | 835 |
| QQL00065.1 | 0.0 | 1 | 835 | 1 | 835 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2JE5_A | 0.0 | 81 | 800 | 1 | 720 | StructuralAnd Mechanistic Basis Of Penicillin Binding Protein Inhibition By Lactivicins [Streptococcus pneumoniae R6],2JE5_B Structural And Mechanistic Basis Of Penicillin Binding Protein Inhibition By Lactivicins [Streptococcus pneumoniae R6] |
| 2BG1_A | 1.53e-288 | 331 | 800 | 25 | 494 | Activesite restructuring regulates ligand recognition in classA Penicillin-binding proteins (PBPs) [Streptococcus pneumoniae R6],2XD5_A Structural insights into the catalytic mechanism and the role of Streptococcus pneumoniae PBP1b [Streptococcus pneumoniae R6],2XD5_B Structural insights into the catalytic mechanism and the role of Streptococcus pneumoniae PBP1b [Streptococcus pneumoniae R6] |
| 2XD1_A | 1.53e-288 | 331 | 800 | 25 | 494 | ACTIVESITE RESTRUCTURING REGULATES LIGAND RECOGNITION IN CLASS A PENICILLIN-BINDING PROTEINS [Streptococcus pneumoniae R6],2XD1_B ACTIVE SITE RESTRUCTURING REGULATES LIGAND RECOGNITION IN CLASS A PENICILLIN-BINDING PROTEINS [Streptococcus pneumoniae R6] |
| 2Y2G_A | 3.07e-288 | 331 | 800 | 25 | 494 | Penicillin-BindingProtein 1b (Pbp-1b) In Complex With An Alkyl Boronate (A01) [Streptococcus pneumoniae R6],2Y2G_B Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (A01) [Streptococcus pneumoniae R6],2Y2H_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Za2) [Streptococcus pneumoniae R6],2Y2H_B Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Za2) [Streptococcus pneumoniae R6],2Y2I_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Za3) [Streptococcus pneumoniae R6],2Y2J_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Za4) [Streptococcus pneumoniae R6],2Y2K_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Za5) [Streptococcus pneumoniae R6],2Y2L_A Penicillin-binding Protein 1b (pbp-1b) In Complex With An Alkyl Boronate (e06) [Streptococcus pneumoniae R6],2Y2L_B Penicillin-binding Protein 1b (pbp-1b) In Complex With An Alkyl Boronate (e06) [Streptococcus pneumoniae R6],2Y2M_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (E08) [Streptococcus pneumoniae R6],2Y2N_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (E07) [Streptococcus pneumoniae R6],2Y2O_A Penicillin-binding Protein 1b (pbp-1b) In Complex With An Alkyl Boronate (eo9) [Streptococcus pneumoniae R6],2Y2P_A Penicillin-binding protein 1b (pbp-1b) in complex with an alkyl boronate (z10) [Streptococcus pneumoniae R6],2Y2Q_A Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Z06) [Streptococcus pneumoniae R6],2Y2Q_B Penicillin-Binding Protein 1b (Pbp-1b) In Complex With An Alkyl Boronate (Z06) [Streptococcus pneumoniae R6] |
| 2UWX_A | 8.76e-288 | 331 | 800 | 25 | 494 | Activesite restructuring regulates ligand recognition in class A penicillin-binding proteins [Streptococcus pneumoniae R6] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8XJ01 | 3.90e-54 | 69 | 698 | 25 | 651 | Penicillin-binding protein 1A OS=Clostridium perfringens (strain 13 / Type A) OX=195102 GN=pbpA PE=3 SV=1 |
| Q0SRL7 | 2.84e-53 | 69 | 698 | 25 | 651 | Penicillin-binding protein 1A OS=Clostridium perfringens (strain SM101 / Type A) OX=289380 GN=pbpA PE=3 SV=1 |
| Q0TNZ8 | 7.67e-53 | 69 | 698 | 25 | 651 | Penicillin-binding protein 1A OS=Clostridium perfringens (strain ATCC 13124 / DSM 756 / JCM 1290 / NCIMB 6125 / NCTC 8237 / Type A) OX=195103 GN=pbpA PE=3 SV=1 |
| P38050 | 4.60e-52 | 115 | 712 | 49 | 597 | Penicillin-binding protein 1F OS=Bacillus subtilis (strain 168) OX=224308 GN=pbpF PE=2 SV=2 |
| P39793 | 2.79e-51 | 29 | 749 | 8 | 671 | Penicillin-binding protein 1A/1B OS=Bacillus subtilis (strain 168) OX=224308 GN=ponA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
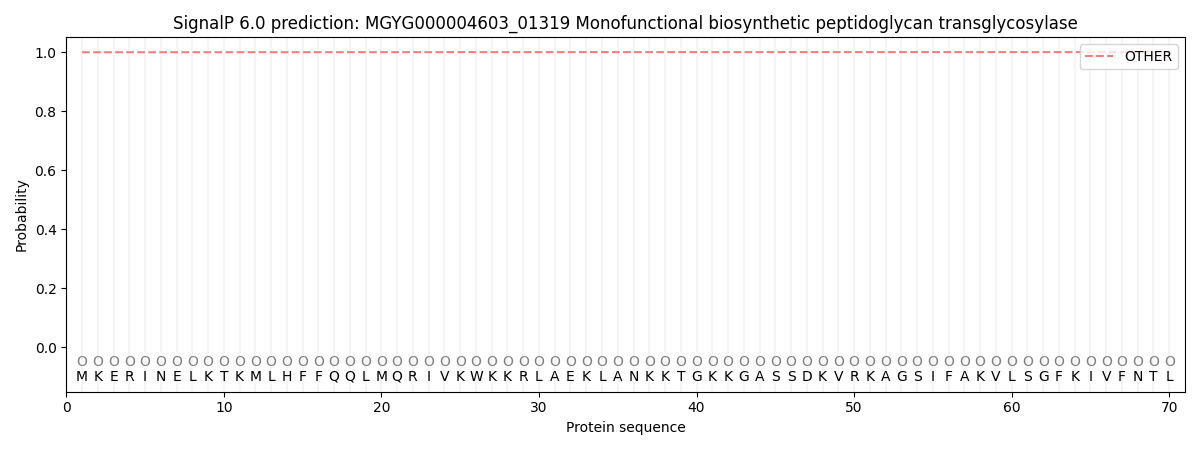
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.999549 | 0.000401 | 0.000026 | 0.000005 | 0.000001 | 0.000009 |